Annals of Pharmacotherapy, Ahead of Print.
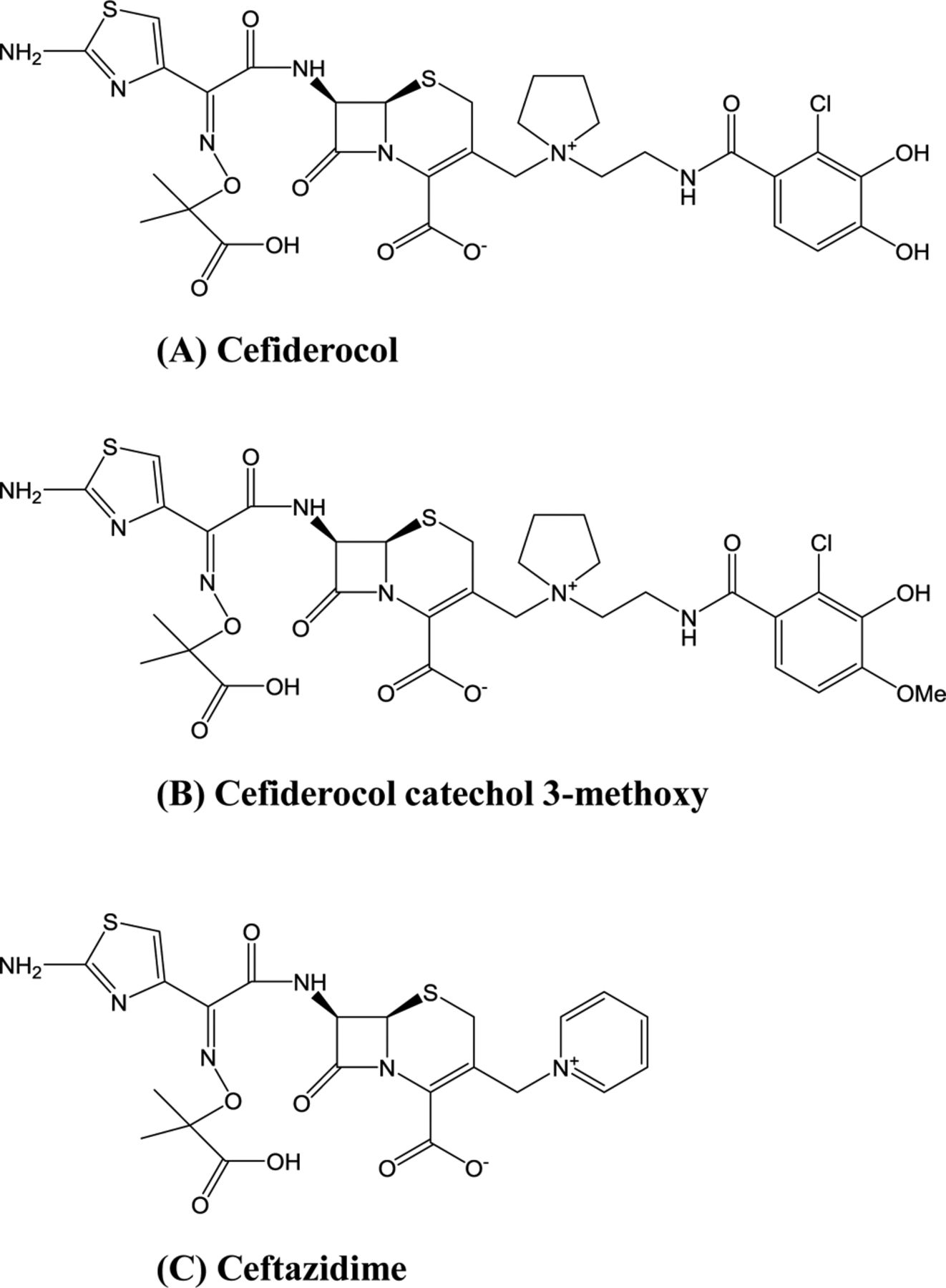
Objective: This article reviews the available data on the chemistry, spectrum of activity, pharmacokinetic and pharmacodynamic properties, clinical efficacy, and potential place in therapy of cefiderocol. Data Sources: A literature search through PubMed, Google Scholar, and ClinicalTrials.gov was conducted (2009 to March 2020) using the search terms cefiderocol and S-649266. Abstracts presented at recent conferences, prescribing information, and information from the US Food and Drug Administration (FDA) and the manufacturer’s website were reviewed. Study Selection and Data Extraction: All relevant published articles, package inserts, and unpublished meeting abstracts on cefiderocol were reviewed. Data Synthesis: Cefiderocol is the first siderophore antibiotic to be approved by the FDA. It was shown to be active against a wide range of resistant Gram-negative pathogens, including multidrug-resistant (MDR) Pseudomonas aeruginosa, Acinetobacter baumannii, Enterobacteriaceae, and Stenotrophomonas maltophilia. Cefiderocol was studied in the treatment of adult patients with complicated urinary tract infections (cUTIs) and nosocomial pneumonia and was well tolerated. In a recently completed prospective study, higher mortality was observed with cefiderocol in the treatment of serious infections caused by carbapenem-resistant (CR) Gram-negative pathogens. Relevance to Patient Care and Clinical Practice: The approval of cefiderocol provides a new option in the treatment of cUTIs and potentially treatment of nosocomial pneumonia caused by resistant Gram-negative pathogens. Given the higher mortality observed with cefiderocol, its use in the treatment of CR Gram-negative infections should be carefully considered. Conclusion: Cefiderocol shows promising activity against MDR Gram-negative pathogens. Its use in the treatment of serious infections caused by CR Gram-negative bacteria needs further evaluation in phase III clinical studies.
Objective: This article reviews the available data on the chemistry, spectrum of activity, pharmacokinetic and pharmacodynamic properties, clinical efficacy, and potential place in therapy of cefiderocol. Data Sources: A literature search through PubMed, Google Scholar, and ClinicalTrials.gov was conducted (2009 to March 2020) using the search terms cefiderocol and S-649266. Abstracts presented at recent conferences, prescribing information, and information from the US Food and Drug Administration (FDA) and the manufacturer’s website were reviewed. Study Selection and Data Extraction: All relevant published articles, package inserts, and unpublished meeting abstracts on cefiderocol were reviewed. Data Synthesis: Cefiderocol is the first siderophore antibiotic to be approved by the FDA. It was shown to be active against a wide range of resistant Gram-negative pathogens, including multidrug-resistant (MDR) Pseudomonas aeruginosa, Acinetobacter baumannii, Enterobacteriaceae, and Stenotrophomonas maltophilia. Cefiderocol was studied in the treatment of adult patients with complicated urinary tract infections (cUTIs) and nosocomial pneumonia and was well tolerated. In a recently completed prospective study, higher mortality was observed with cefiderocol in the treatment of serious infections caused by carbapenem-resistant (CR) Gram-negative pathogens. Relevance to Patient Care and Clinical Practice: The approval of cefiderocol provides a new option in the treatment of cUTIs and potentially treatment of nosocomial pneumonia caused by resistant Gram-negative pathogens. Given the higher mortality observed with cefiderocol, its use in the treatment of CR Gram-negative infections should be carefully considered. Conclusion: Cefiderocol shows promising activity against MDR Gram-negative pathogens. Its use in the treatment of serious infections caused by CR Gram-negative bacteria needs further evaluation in phase III clinical studies.