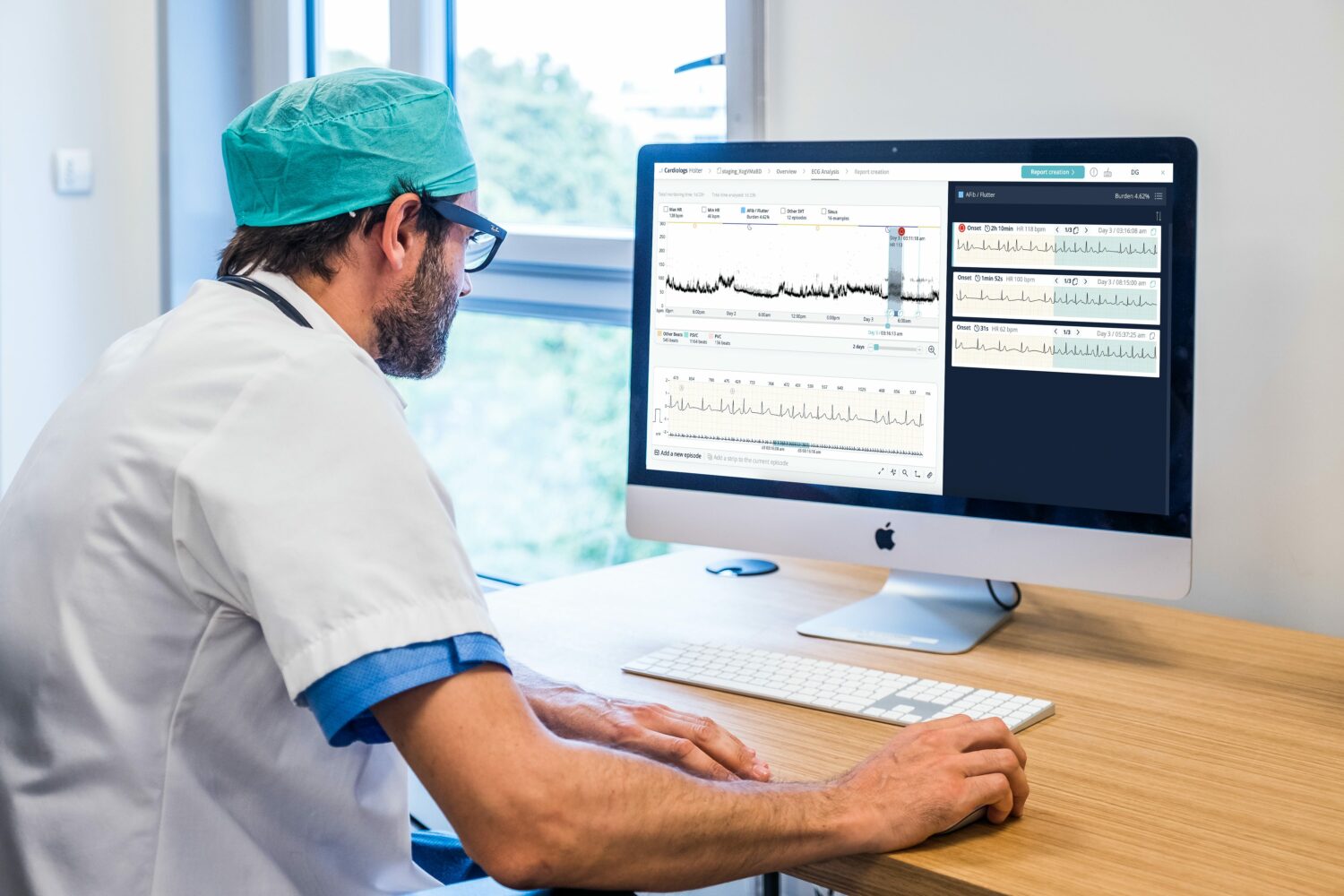
What You Should Know:
Cardiologs announced today that the FDA has cleared their algorithm to be used for the pediatric population – younger than 18. Their technology can now analyze ECG readings for atrial arrhythmias across all age groups.
Cardiologs, a medical technology company committed to transforming cardiac diagnostics using medical-grade artificial intelligence and cloud technology, announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to use its AI-powered cardiac diagnostics platform for pediatric cardiology.
Supporting More Than 40 Million EKG Recordings
The new model, now supported by more than 40 million EKG recordings, was used to evaluate a global sample of 10,000 EKG readings from patients in various age groups. Results showed that the updated algorithm improved sensitivity by 14% while reducing the number of false positives by 48% for all major arrhythmias. In addition, arrhythmias detection performance was equal across all age groups, regardless of the EKG recording device.
Recent Traction
This is the latest in a string of recent milestones for Cardiologs. Earlier this month, the company announced the results of a new study that showed its AI model can detect atrial arrhythmias (AAs) better than the Apple Watch’s electrocardiogram (ECG) algorithm. News also broke that Cardiologs is being purchased by healthcare technology leader Royal Philips, which plans to use its global footprint to accelerate the adoption of Cardiologs’ technology worldwide.