The growing pipeline of monoclonal antibodies, vaccines, enzymes, biosimilars and other therapeutic proteins have created an ever- increasing demand for highly- productive and stable cell lines. Many of the recombinant biotherapeutic products, produced in cellular systems, have been translated into commercial successes; these include ALPROLIX®, ELOCTATE® and ELAPRASE®. Given their impact on the overall quality of therapeutic protein, it is critical to carry out cell line characterization, at different stages of biopharmaceutical production, to ensure the purity and origin of the producer cell line. In addition to biopharmaceutical development, evaluating quality of in- vitro cellular models is mandatory during toxicology and other R&D studies, to avoid misinterpreted experimental results and delays in research timelines. The requirement of cost intensive material, complicated development and purification processes, expensive characterization and documentation procedures, the need for specialized technologies / infrastructure for the production and storage of cell lines and emergence of advanced analytical techniques, have led to an increase in outsourcing in this domain. Recent years have witnessed the emergence of a large number of highly qualified contract research organizations (CROs) and contract manufacturing organizations (CMOs) that assist drug developers and strive to expedite cell-line related product / process development and biomanufacturing.

Given the effort to halt the COVID-19 pandemic, we are led to believe that one of the contributing factors towards the growth of cell line development and characterization services market is the expertise available with the service providers to develop genetically engineered cell lines, which could serve as potential protein producing factories, for large scale commercialization of the vaccine against the SARS-CoV-2.
Players Offering Cell Line Development and Characterization Services
Presently, over 220 companies / organizations across the globe claim to offer services for cell line development from a variety of sources, to support cell-based R&D operations and facilitate bio-therapeutics production. As per our research, close to 80% of these players additionally offer analytical and purification services for proteins, in order to ensure the quality and purity of biologics produced from cell lines. Examples of players, which have established complete suite of services and serve as one stop shop for drug developers, include (in alphabetical order) Lonza, Samsung Biologics and WuXi Biologics.
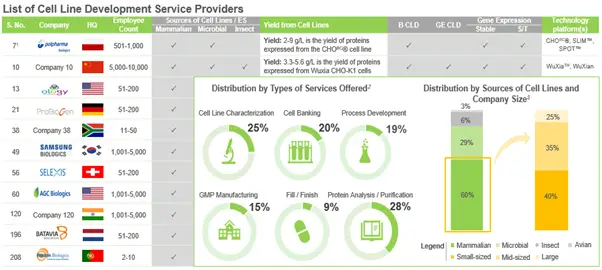
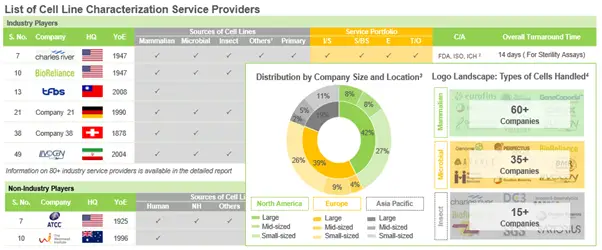
In addition to this, we identified over 80 companies that offer cell line characterization services; around 76% of these characterize mammalian cell lines, followed by companies that characterize microbial cells (41%). Examples of players characterizing mammalian, microbial and insect cells, include (in alphabetical order) Accelero Bioanalytics, Eurofins Genomics, LivoGen Pharmed and SGS Life Sciences. In addition to this, several non- industry players have also taken significant initiatives in this domain.
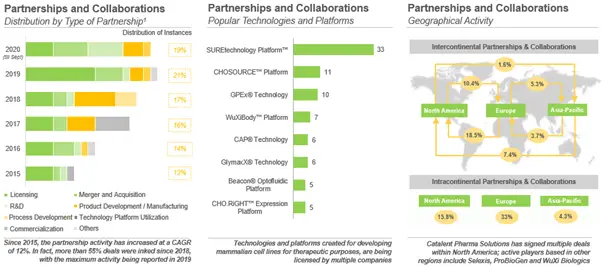
Which Type of Deals are being Inked in this Domain?
Majority of the collaborations (23%), inked by the stakeholders in this domain, were focused on licensing of cell lines / models, biologics and / or technology platforms related to cell lines. Examples of the recently signed licensing deals include (in reverse chronological order) collaboration between Horizon Discovery and TrueBinding (July 2020), Horizon Discovery and Rentschler Biopharma (May 2020) and CEVEC Pharmaceuticals and Evox Therapeutics (April 2020). In the second and third quarter of 2020, the service providers entered into a number of agreements related to the development of vaccine against SARS-CoV-2, signifying the utility of cell lines for the treatment of infectious diseases; examples of deals inked for the development of vaccine against COVID-19 include (in reverse chronological order) collaboration between Northway Biotechpharma and Memo Therapeutics (August 2020), Catalent Pharma Solutions and Spicona (June 2020), Aragen Bioscience and Oragenics (May 2020).
Likely Growth of the Cell Line Development and Characterization Services Market
During our research, we estimated the market under conservative, base and optimistic scenarios. As per the base case forecast scenario, rye market for cell line development services is estimated to be worth over USD 5.3 billion, growing at an annualized rate of 13.7%, in the given time period. The opportunity is likely to be well distributed across different geographies, cell lines sources, applications and company sizes.
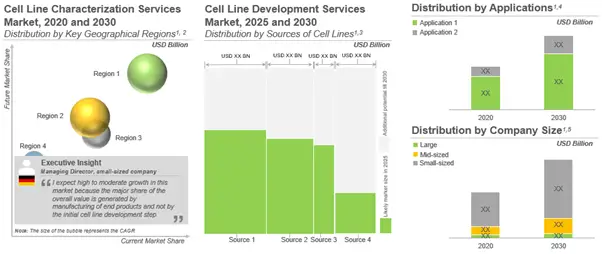
Further, the cell line characterization services market is expected to witness a CAGR of nearly 13%. Presently, majority of revenues is generated from projects related to the characterization of mammalian cell lines (88%). The trend is unlikely to change in the foreseen future.
Cell Line Development and Characterization: What’s New?
The technical aspect of this field is witnessing a lot of innovation. In the recent years, the evolution of CRISPR and other gene editing technologies, has led to the development of customized knock-in, knockout and protein over-expressing cell lines. Notable examples of companies developing such designer cell lines include (in alphabetical order) Creative Biogene, Creative Biomart and Syd Labs. Further, many players have developed their own cell- based novel and innovative technologies, to lower manufacturing costs and production timelines. Examples of such companies include (in alphabetical order) Abzena (Composite CHO technology), AGC Biologics (CHEF1® expression platform) Batavia Biosciences (STEP® technology), Celltheon (SMART
technology), AGC Biologics (CHEF1® expression platform) Batavia Biosciences (STEP® technology), Celltheon (SMART expression platform), Celonic (CHOvolution
expression platform), Celonic (CHOvolution / SEFEX platform), Cevec Pharmaceuticals (CAP®GO platform) and Selexis (SUREtechnology Platform
/ SEFEX platform), Cevec Pharmaceuticals (CAP®GO platform) and Selexis (SUREtechnology Platform ).
).
Having said that, we believe that in pursuit of exploring “an ocean of opportunities”, these service providers will continue to contribute to the technological evolution in this domain, in the foreseen future!
For detailed insights about this domain, check out our report.
Cell Line Development and Characterization Services
You may also be interested in the following titles:
- HUMIRA® (Adalimumab) Biosimilars: Focus on Approved & Launched Biosimilars, Investigational & Research Use Biosimilars, Inactive / Terminated / Withdrawn Biosimilars, Industry / Non-Industry Partnerships
- CRISPR Based Therapeutics Market, 2021-2030
- Display Library Technologies and Affiliated Services Market, 2021-2030
The post Cell Line Development and Characterization: An Ocean of Opportunities for Service Providers appeared first on Blog.