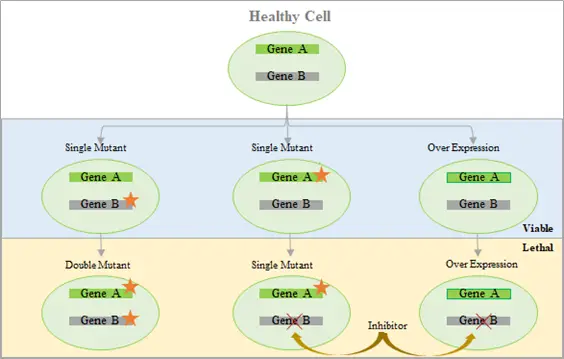
Cancer is considered to be the second leading cause of mortality, after cardiovascular diseases, accounting for every sixth reported death in the world. The International Agency for Research on Cancer (IARC) states that the number of new cancer cases is expected to grow to 27.5 million across the globe, by 2040. Experimental evidence has shown that defects in the deoxyribonucleic acid (DNA) repair machinery are one of the primary causes for both generation and maintenance of cancer. It is a well-known fact that DNA is the reservoir of genetic information in all cells. Defects in deoxyribonucleic acid (DNA) repair have been shown to be one of the primary causes of cancer. Moreover, tumor cells that are characterized by impaired DNA repair pathways typically become reliant on alternative DNA repair pathways for survival. This phenomenon is commonly referred to as oncogene addiction.
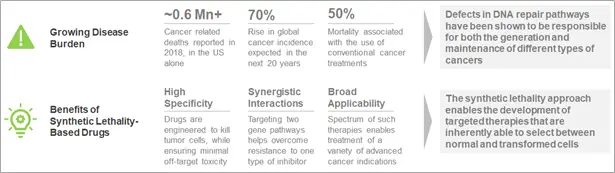
What is the Basic Concept of Synthetic Lethality?
Inhibitors of such compensatory repair pathways have the potential to sensitize cancer cells to DNA damaging agents and other therapeutic regimens. On the other hand, the simultaneous inactivation of certain pairs of genes have been shown to cause cell death. This phenomenon is known as synthetic lethality. In cancers, where mutations have led to the loss of function of one gene, using a drug molecule that specifically targets the corresponding gene of the synlet pair has been demonstrated to be a viable and effective therapeutic regimen.
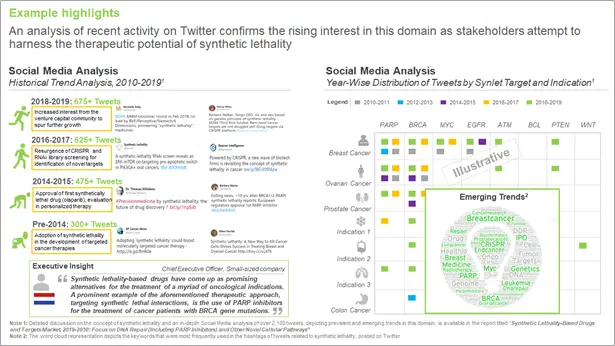
What is the recent activity on Social Media Platforms?
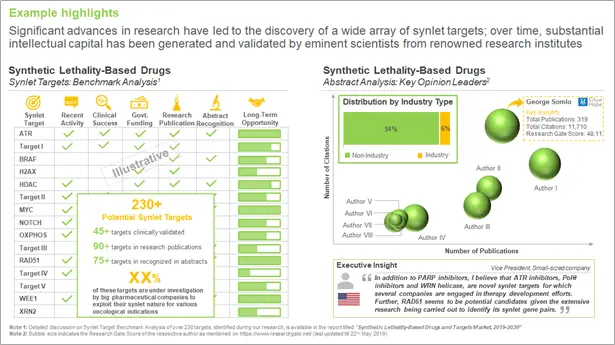
Further, recent activity on Twitter (2,100+ tweets) confirms the rising interest in this domain as stakeholders attempt to harness the therapeutic potential of synthetic lethality. In addition to PARP inhibitors / BRCA mutations, ATM, ATR, CHK1, KRAS and RAD51, are presently considered amongst the most prominent synlet targets, which are being investigated in the clinical stages of development. Further, CDK12, PI3K, PLK1, PTEN, SNF and WRN are synlet targets that are currently being evaluated in preclinical studies.
What are the most popular synlet targets being explored for drug development?
Significant advances in research have led to the discovery of a wide array of synlet targets; over time, substantial intellectual capital has been generated and validated by eminent scientists from renowned research institutes. Specifically, 55+ targets are already clinically / preclinically validated, while over 160 targets have been mentioned in various research publications (with no drug candidate so far).
Recent Approvals: Drug Watch 2020
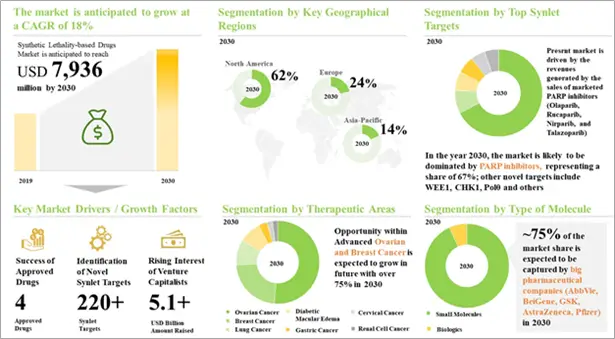
It is worth noting that, till date, only four molecules have been marketed, namely olaparib (2014), rucaparib (2016), niraparib (2017) and talazoparib (2018). Recently, in April 2020, the USFDA approved GSK’s PARP inhibitor, niraparib, for expanded use as a maintenance therapy in advanced ovarian, fallopian tube, or primary peritoneal cancer patients in complete or partial response to frontline platinum-based chemotherapy. In January 2020, the USFDA granted priority review designation to a supplemental new drug application (sNDA) for olaparib as maintenance therapy in advanced ovarian, fallopian tube, or primary peritoneal cancer patients in complete or partial response to frontline platinum-based chemotherapy. In January 2020, NICE recommended olaparib for use in the NHS for BRCA-positive patients in the second-line, post-platinum-chemotherapy treatment setting for ovarian cancer. In January 2020, the USFDA granted fast track designation to Clovis Oncology’s rucaparib for prostate cancer. In addition, two molecules, veliparib (developed by AbbVie) and pamiparib (developed by BeiGene), are in phase III of development and are likely to reach the market in the near future.
How is the current and estimated opportunity segmented across key market segments?
The present market is driven by the sales generated by four approved PARP inhibitors, niraparib, olaparib, rucaparib and talazoparib. Future growth of the market is likely to be driven by the success of clinical outcomes of late-stage molecules; industry stakeholders are optimistic about the vast potential of PARP inhibitors.
To get detailed insights on the key players, synlet targets, funding and investment trends research landscape, recent developments and the likely market evolution, check out the report here
You may also be interested in the following titles:
- Cell and Advanced Therapies Supply Chain Management Market, 2019-2030
- RNAi Therapeutics Market (2nd Edition), 2019 – 2030
- Gene Therapy Market (3rd Edition), 2019 – 2030
The post Rising Popularity of Drug Targeting Synthetically Lethal Targets Fuels the Battle of PARP Inhibitors for Treatment of Advanced Cancer Indications appeared first on Blog.