What You Should Know:
– Propeller Health announced
it will co-package a new asthma medication from Novartis, which was
approved by the European Commission this week for use in the EU.
– Enerzair®
Breezhaler® plus Propeller Health sensor is the first asthma medication to be
co-packaged and co-prescribed with a digital health platform.
– Propeller’s solution
works by attaching a sensor to the Enerzair® Breezhaler® inhaler, which then
delivers objective data on medication use to the Propeller app on the patient’s
smartphone
Propeller Health today announced a collaboration with Novartis to co-packaged the Propeller digital health platform with Enerzair® Breezhaler® (QVM149; indacaterol acetate, glycopyrronium bromide and mometasone furoate [IND/GLY/MF]), a recently approved Novartis medication developed to treat uncontrolled asthma. Propeller previously announced a collaboration with Novartis to develop a custom add-on sensor for the Breezhaler® inhaler, a device used for the company’s portfolio of COPD treatments (Ultibro® Breezhaler®, Onbrez® Breezhaler®, and Seebri® Breezhaler®), connecting these medications to Propeller’s digital health platform. The same sensor will be co-packaged with Enerzair® Breezhaler®.
Why It Matters
This collaboration marks the first time a digital health tool will be packaged and prescribed alongside an inhaled asthma medication. Enerzair® Breezhaler® and Propeller sensor and app received approval from the European Commission in July and will launch across Europe starting in 2020. Healthcare professionals in Europe will have the option to prescribe Enerzair® Breezhaler® with or without the companion digital health platform. The medication is not available in the U.S.
Enerzair® Breezhaler® was approved as a maintenance treatment of
asthma in adult patients not adequately controlled with a maintenance
combination of a long-acting beta2-agonist
(LABA) and a high dose of an inhaled corticosteroid (ICS) who experienced one
or more asthma exacerbations in the previous year.
Impact of Uncontrolled Asthma
Asthma affects an estimated 358 million people worldwide and can cause a significant personal, health, and financial burden when not adequately controlled. Despite current therapy, over 40% of patients with asthma at Global Initiative for Asthma (GINA) Step 3, and over 45% at GINA Steps 4 and 5 remain uncontrolled. Patients with uncontrolled asthma may downplay or underestimate the severity of their disease and are at a higher risk of exacerbation, hospitalization, or death. Barriers, such as less than optimal adherence, incorrect inhaler technique, treatment mismatch, safety issues with oral corticosteroids, and ineligibility for biologics, have created an unmet medical need in asthma.
Enerzair Breezhaler
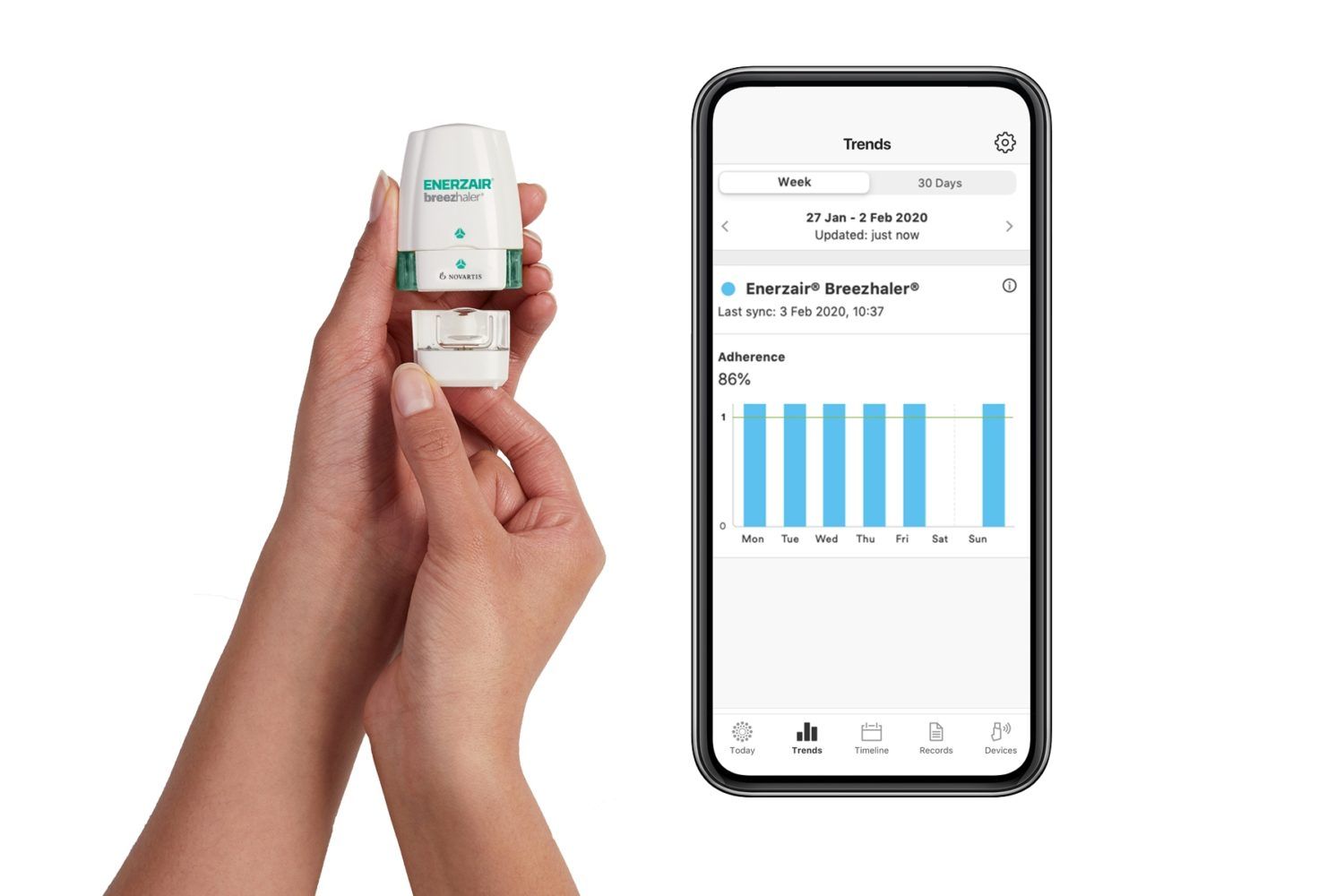
Enerzair Breezhaler is provided in a transparent capsule that allows patients to see that they have taken their medication and will be administered via the dose-confirming Breezhaler® device, which enables once-daily inhalation using a single inhaler. The digital companion includes a sensor that attaches to the Breezhaler device and can be linked to the Propeller Health smartphone app, providing patients with inhalation confirmation, medication reminders, and access to objective data that can be shared with their physician in order to help them make better therapeutic decisions.
Propeller’s solution works by attaching a sensor to the
Enerzair® Breezhaler® inhaler, which then delivers objective data on medication
use to the Propeller app on the patient’s smartphone. The app also sends the
patient reminders to take their prescribed dose and keeps a record of adherence
data over time. The patient can share that data with their clinician to help
inform the patient’s treatment plan.
In previous clinical studies unrelated to this collaboration, the Propeller platform has been shown to increase asthma control by up to 63 percent, increase medication adherence by up to 58 percent, and reduce asthma-related emergency department visits and hospitalizations by as much as 57 percent.
“Our collaboration with Novartis to co-package Propeller with Enerzair® Breezhaler® is the first time a pharmaceutical company and digital health company have worked together to package a digital health platform with an asthma medication,” said David Van Sickle, co-founder and CEO of Propeller Health. “The ability to prescribe a maintenance medication with Propeller will make it easier for healthcare professionals to engage their patients in self-management.”