What You Should Know:
– Today, Respira is introducing its AI wearable technology to monitor for certain very serious lung conditions. According to the Institute for Health Metrics and Evaluation, COPD (Chronic Obstructive Pulmonary Disease) and COVID-19 were the 3rd and 4th global causes of death during the height of the pandemic. Asthma, according to the World Health Organization, is the most common chronic disease among children.
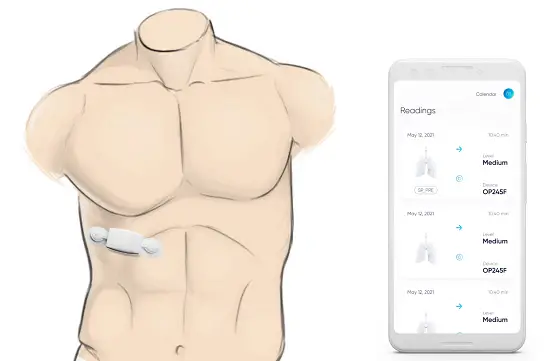
– Chest wearable uses tiny speakers and microphones to inject noise in lungs and ‘listen’ for lung resonance changes to detect unhealthy lung air trapping.
Respira Labs, a Mountain View, CA-based medical technology company specializing in respiratory care, today announces a new wearable that continuously and easily assesses lung function, without requiring patients to blow into anything. Designed initially to assess COPD, COVID-19 and asthma patients, the wearable patch has embedded speakers and microphones which measure changes in acoustic resonance as a proxy for changes in lung air volume, the basis of pulmonary function testing.
Remotely Monitoring COPD, COVID-19 and Asthma Patients at Home
The chest wearable, called Sylvee, is worn by patients on the lower part of the rib cage. It is paid for by Medicare and monitors lung function over time to provide a comprehensive overview of a patient’s condition. Sylvee ‘injects’ noise into the lung area and then measures the type of sound that is produced. Like a thump on a drum, if there is air trapped in the lung the sound it makes differs from the resonance of sound produced when air is fully expelled from the lungs. Air trapping is a central and early symptom of respiratory decline. The Sylvee app uses DSP (digital signal processing) and AI to analyze the results which pulmonologists and primary care physicians can review, looking specifically at lung volume, capacity, rates of flow and trapped air.
Sylvee Accuracy
Respira Labs has set a goal of achieving 90% accuracy in measuring air trapping by pursuing a large trial of more than 500 patients located both in the U.S. and internationally. They also intend to publish in top journals by late 2022. The device is currently in prototype with FDA clearance expected within the next 18 months.
“Well-established science shows that air trapping can be measured with more than 90% accuracy using low-frequency sound. There is a clear difference in the acoustic resonance spectra of COPD patients versus healthy controls,” explained Dr. Maria Artunduaga, Respira Lab’s founder and CEO. “With more than 100 million Americans affected by COPD, COVID-19 and asthma, and with an aging population, it can be lifesaving to remotely and accurately monitor lung function and discover a problem early enough to avoid serious consequences. Our goal is to flag abnormalities early, enable earlier treatment at home and empower patients to manage their own health.”