Patient adherence to therapy continues to be a challenge for pharmaceutical manufacturers. New complex patient journeys and multi-channel brand distribution models make it increasingly difficult to address patient barriers to adherence. Manufacturers continue to spend millions of dollars on patient services and adherence programs to keep patients on therapy with often little return. In fact, estimates show that up to half of all prescribed medications are not taken as prescribed and some 20% to 30% of all persistent medication prescriptions are never filled.1,2 This costs the US life sciences industry more than $250 billion3 in revenue and an additional $290 billion in avoidable healthcare costs annually.4
Most manufacturers heavily invest in patient services programs to increase adherence. These programs can be costly and might or might not be effective—it is simply unknown because most manufacturers do not have adequate success criteria nor true understanding of the effectiveness of the programs.
The struggle for effective patient services programs
Implementing effective patient services programs to increase patient adherence is very challenging for many manufacturers. To start, many patient services initiatives often are siloed and uncoordinated. These programs typically are executed by multiple departments for multiple brands utilizing both internal teams and external partners. This uncoordinated approach can be inefficient and costly. The result is that patient services providers can unknowingly approach the same patient with different programs, causing both confusion and in some cases offsetting the programs’ desired effect completely.
Another barrier to effectiveness is a lack of data governance and stewardship related to patient services. Patient services data is often unstructured and lacking when it comes to data governance and stewardship. Manufacturers too often leave data quality, format, and mastering to chance when receiving data from the patient services providers. This causes many problems, including the inability to analyze the program’s success.
Few manufacturers have determined how to best predict and measure program costs. Generally, cost prediction is done at the aggregate level for multiple programs and in annual buckets, and there is no clear line of sight to what an individual patient services program costs. In addition, most manufacturers typically do not have the ability to predict or trend costs for patient services. This means they are forced to react to actual cost changes and adjust budgets in real time as the actual costs come in monthly from the patient services providers.
Further to the challenge of measuring costs, measuring patient services program effectiveness is often unstructured and lacks clear success criteria. Manufacturers will utilize anecdotal evidence for program measurement with little or no control criteria. It is increasingly uncommon for a manufacturer to establish and utilize formal success criteria for patient services programs prior to initiation and conduct ongoing measurement and evaluation of their program goals.
Lastly, most manufacturers do not employ advanced analytics and prediction regarding their services programs and investments in this area. The employment of artificial intelligence/machine learning techniques to predict both risk and intervention strategy is underutilized and rarely deployed.
Patient services strategies
While it is not the deep focus of this article, it is good to review strategies for excellence for patient services programs. First, manufacturers need to understand the full scope of these services broadly employed across their organization. This means that each patient services program should be defined, including the purpose, the program owner, the duration, the target, the timing of targeting, the success criteria, and the projected costs. Next, stakeholders should analyze and determine how patient services should be managed in their organizations. Should they be centralized—organizationally deploying all patient services from one department—or should the manufacturer utilize a patient services Center of Excellence that meets on a recurring basis to coordinate? And lastly, manufacturers ideally should name an approval council. This council would be charged with developing standards of selection, implementation, and management of the programs. Every patient services program should be held to a high standard and should meet a defined set of criteria prior to implementation, and the patient services approval council would be tasked with reviewing and approving each program.
Patient services data quality and data governance
 It is hard to stress how critical data governance and stewardship are when deploying patient services programs to measure adherence. When properly deployed, data quality and data governance programs allow program owners to truly measure, evaluate, and eventually predict their program services for their target patient population.
It is hard to stress how critical data governance and stewardship are when deploying patient services programs to measure adherence. When properly deployed, data quality and data governance programs allow program owners to truly measure, evaluate, and eventually predict their program services for their target patient population.
When establishing data quality and data governance programs, there are some key data strategies to consider.
Consistent data modeling and metadata. Often, patient services stakeholders do not think of the details required for quality data—including data layouts, value types, and descriptors—which can require extensive thought and design. However, when this critical foundational task is skipped, the results can be disastrous. Imagine deploying a patient services program across multiple specialty pharmacies, each of which delivers data in a different format with different values describing a given program. With these disparate datasets, it is nearly impossible to report on results across these specialty pharmacies, much less accurately evaluate the success of the program. More data-centric manufacturers will require a strategic planning process that creates a strong data model, taking into account current and possible future needs as well as metadata that defines what columns mean and how they should be used.
Data quality and stewardship. The lack of accuracy of data values reported by patient services providers can be pretty grim at times. It is critical for manufacturers to create processes that can steward and triage these errors. The most common data quality checks that should be standard protocol include:
- Null checks: Null or blank values for key data points is very common. Manufacturers should be able to trap and identify these errors and provide timely feedback to data providers.
- Valid entry: Values provided by data providers can be ungoverned and inconsistent making reporting and analytics difficult. Manufacturers should utilize internal control metadata to verify all reported data elements.
- Event timing: Patient services execution or timing is often critical for the service engagement success. Measuring the timing of a patient services will highlight if patient services are completed within the window of time for execution (for example, within two days of a new referral to be issued a patient welcome kit).
- Event sequencing: Patient services are often executed in predefined sequence. Checking for sequencing is a more sophisticated check but is required to ensure that patient services are executed in sequence (for example, first a welcome kit, followed by patient education, an adherence call, etc.).
IntegriChain recently surveyed the data quality and stewardship of patient services data delivered to dozens of manufacturers, finding some alarming results that impact patient adherence.
- Timely interventions: Patient services programs are often designed to be executed within a specific time period from a patient journey event. An example of this would be a patient welcome kit, which is designed to be delivered within a tight window following patient initiation. A survey of manufacturer interventions revealed that only 48% of interventions are executed within the agreed upon SLA.
- Missed interventions: Patient services interventions typically have both a timeframe as well as a specific target patient case. Manufacturers very rarely have the ability to measure patient interventions that are actually executed versus how many should have been performed. A survey of manufacturer interventions showed a GAP of 28% missed interventions.
- Sequenced interventions: Patient services interventions will often have a desired sequence for execution delivery. A patient would not expect to get an adherence call prior to a welcome kit, so service sequence can be important to the patient experience. A survey of manufacturer interventions highlighted that only 55% of interventions were executed in the correct sequence.
Data mastering. Data mastering strategies can have a large impact on patient services data. Although mastering is part of the data stewardship umbrella, it requires more data skills and infrastructure. The key data elements of mastering include product and diagnostic codes, provider, and payer and channel. For most patient services programs to be analyzed or measured, manufacturers will need to connect the patient services activity data with patient status and/or hub data, which can be a significant challenge.
Strategies for mastering patient challenges
Manufacturers need to map patient services activities to traditional patient status data to measure the full impact of these services on the brand patient journey. Manufacturers also should utilize key performance indicators such as Time on Therapy or Proportion of Days Covered in comparison with the patient services deployed to measure program success and impact. And lastly, they will need a data strategy for connecting their patient services activities to patient status or patient journey data to fully analyze their program performance.
Perhaps the most critical mastering data element is the patient identifier. Without aligning these data sets on a single identifier, manufacturers are unable to answer critical questions including: What percentage of patients in my specialty pharmacy network utilize patient services programs? Are my patients who utilize my patient services more likely to stay on therapy longer?
Patient identifiers are key to mapping a patient journey. Stewarding and connecting the patient identifiers across all the disparate data providers can be challenging. Figure 1 illustrates an example of these challenges and methodologies to overcome them.
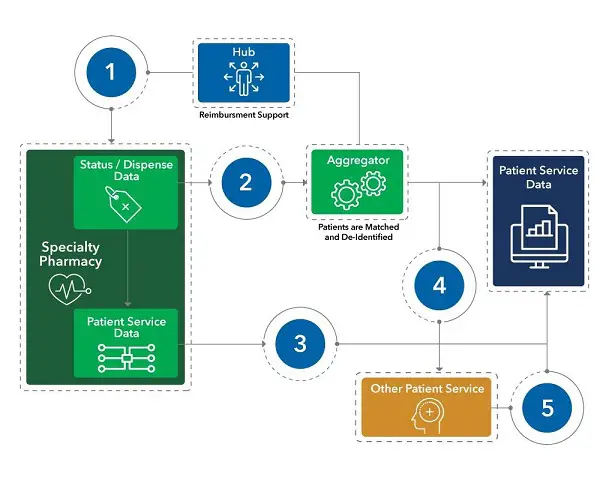
Figure 1. Strategies to overcome patient mastering challenges.
1. Patient cases that are transferred from a hub to a specialty pharmacy can lose their longitudinal connection. This causes the specialty pharmacy to create a new longitudinal ID for the patient, essentially restarting the patient journey. Advanced stewardship techniques can be utilized to identify patients who are lost during the transfer process. Secondary patient matching techniques can be deployed to match existing patient cases with new cases.
2. Specialty pharmacies can report patient cases with null identifiers or even patient cases that have multiple identifiers. Data stewardship checks should triage for multiple specialty pharmacy identifiers as well as for any reported as null. These checks should be completed daily, and any errors should be corrected before they can impact patient journey analytics.
3. Patient services data reported by specialty pharmacies often will not be sent to the aggregator, and therefore will not have a longitudinal patient ID. Specialty pharmacy patient services data will need to be matched back to a longitudinal ID by matching the specialty pharmacy IDs for the patient case.
4 & 5. Patient services providers will rarely consume key patient identifiers prior to managing patient cases and as a result, will not report back service activity with a patient identifier that can be used to create patient journey insights. Patient services providers will need to be fed the longitudinal ID for any patient case that they triage. This key should be reported back by the patient services provider to guarantee patient longitudinality.
Measuring patient services program effectiveness
The criteria for measuring a patient services program should be established before execution. Program evaluation should be done on a recurring basis, often every six months. One of the first steps is to establish program measurement criteria as well as measurement intervals. Manufacturers should establish explicit review criteria after each evaluation event, allowing them to adjust the program messaging and delivery (see Figure 2). Program directors can also choose to stop the program all together if they are proving to not be effective.
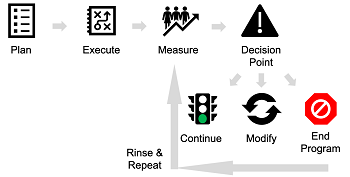
Figure 2. Measuring patient services effectiveness.
Patient services effectiveness impact
IntegriChain has collected data through a survey of various patient services programs. Highlighted below are two cases in which patient services programs had a significant impact on the patient journey.
- 3x days on therapy: A survey of manufacturer interventions revealed a service program produced three times the days of therapy compared with patients who did not receive the targeted patient services program.
- 2x discontinuation rate: Another survey of manufacturer interventions highlighted an example in which patients who did not receive a patient services intervention had a discontinuation rate that was two times greater than patient who did receive the patient services program.
Getting control of costs
With the right data and analytics, manufacturers can better manage patient services costs. The ability to effectively forecast program costs prior to launch allows for more accurate budgeting and accrual predictions both mid and end of year. Additionally, analytics that track spend trends against program effectiveness allow program owners to determine true program cost effectiveness.
Figure 3 below shows an example of a manufacturer’s patient services program forecast trends. Such trend analytics allow more informed spend decisions based on program and brand execution. This example highlights that the manufacturer has underestimated patient services demand. This can be caused by higher brand demand or higher patient services demand. The root cause should be identified and planned for by the patient services owner.
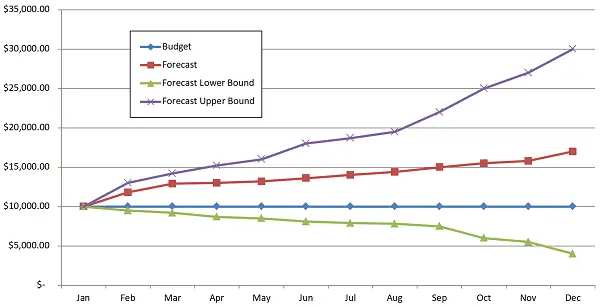
Figure 3. Patient services cost analysis.
Utilizing advanced analytics and machine learning for patient services optimization
Advanced analytics and/or supervised machine learning is definitely not one size fits all for patient services analytics. While there are many inconsistencies across predictive models, they are often brand specific.
First, manufacturers must establish stewardship and governance practices prior to attempting to build predictive models. Key feature sets such as geography, payer, channel, specialty pharmacy, healthcare practitioner, and patient services information must be clean and consistent. When building a predictive model, manufacturers must commit to an iterative process, allowing the supervised portion of the model building to take shape. For most manufacturers, insights focus on three primary business cases for adherence.
- Predicting who needs intervention. This insight is risk scoring: who might stall in initiation or who might opt out of a patient services program.
- Predicting who will cancel or gap/discontinue therapy. These are the most frequently utilized models.
- Predicting the best time to intervene. This model is about timing; once an event has been predicted, when is the best time to intervene with the patient to maintain therapy adherence?
However insightful predictive analytics might be, they mean nothing if they do not trigger action. Manufacturers must be prepared to deploy these insights to the field. Often, this involves piloting test and control initiatives that not only prove the prediction but also test the effect of taking action to prevent the prediction.
Digging deeper into developing predictive models
To develop predictive models that provide actionable insights, collaboration is required among the manufacturer, subject matter experts, and data scientists to understand and identify patient journey barriers that can both be labeled using the available datasets. Additionally, if identified and known ahead of time, support functions responsible for ingesting the predictions and attempting to prevent their occurrence should join the planning work. From a technical standpoint, this is the process of defining the actionable-dependent variables. The next critical step is to explore hypotheses regarding potential factors that can cause the identified patient journey barriers to occur to inform model development.
Data scientists then begin to build preliminary models: one predicting the probability of a new patient opting out of patient services, and the second predicting the likelihood of an active patient having gap days in their next shipment. Significant time is devoted to exploring available features in the data and using the hypotheses discussed above to engineer new features, all in an effort to feed the algorithms enough relevant information so that accurate predictions can be made.
Once preliminary models that achieve accuracy scores significantly greater than flipping a coin are achieved, collaboration resumes on the model output and sample patient journeys that resulted in false positives or false negatives to further hone the model. Ideally, this collaboration and agile development leads to improved model accuracy.
Lastly, once the models are scalable and ready for production, they are deployed alongside pilot programs with the specialty pharmacies. During this pilot program, the manufacturer can use the results of the models—for example, identified high-risk patients—to triage patient services interventions and keep patients on therapy.
Key takeaways
In summary, there are a number of strategic best practices that manufacturers should follow regarding measuring and understanding the impact of patient services programs on the brand patient journey.
Coordinate all patient services programs. Not only should manufacturers understand who the program is reaching, how often, what is the cost, and what defines success, but they also need to understand how multiple programs interact with each other.
Govern the patient services programs. Manufacturers must create data governance rules and processes that will ensure high quality patient services information.
Control patient services costs. Patient services programs can be costly. Manufacturers should understand what a program will cost prior to launch and create capabilities that will predict costs throughout the year.
Define and measure. Each patient services program should have a definition of success that can be measured and expressed. Manufacturers should regularly evaluate patient services programs and continue, tune, or stop the program as needed.
Advanced analytics. Manufacturers should design advanced analytic techniques to not only identify patient cases that are most aligned to their patient services programs but also identify key patient journey intervention points.
About the author
Sean McCarthy is Segment Leader, Patient Access, and Ed Doran is Solutions Manger; both with IntegriChain.
References
- Peterson AM , Takiya L , and Finley R . Meta-analysis of trials of interventions to improve medication adherence. Am J Health Syst Pharm. 2003;60:657-65.
- Haynes RB , Ackloo E , Sahota N , McDonald HP , and Yao X . Interventions for enhancing medication adherence. Cochrane Database System Rev. 2008;2:11
- Medication Adherence: Pharma’s $637 Billion Opportunity, https://healthprize.com/blog/medication-adherence-pharmas-637-billion-opportunity/.
- New England Healthcare Institute, Thinking Outside the Pillbox: A System-wide Approach to Improving Patient Medication Adherence for Chronic Disease. August. 12, 2009, https://www.nehi.net/writable/publication_files/file/pa_issue_brief_final.pdf
The post Strategies for effective patient services programs appeared first on Pharmaceutical Commerce.