
What You Should Know:
– Today, Talis Biomedical Corporation announced it has
been awarded a $25M contract from the National Institutes of Health (NIH) as
part of Phase 2 of its Rapid Acceleration of Diagnostics (RADx) initiative as
well as another $100 million in additional financing.
– The new funding will be used to scale manufacturing for
the launch of the Talis One™ diagnostic platform that provides rapid and highly
accurate detection of COVID-19 at points-of-care in 30 minutes or less.
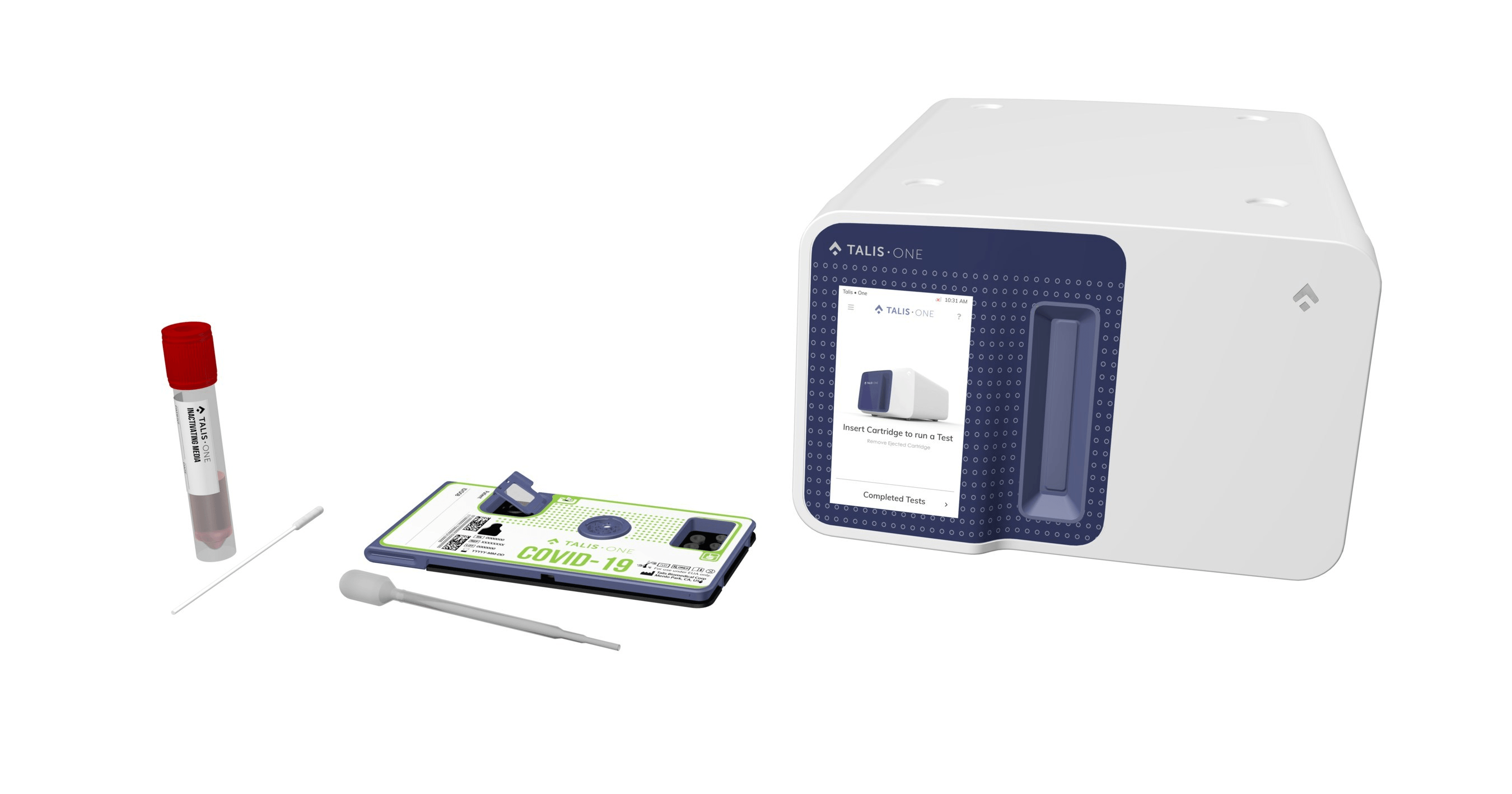
– The Talis One System is a fast, easy-to-use, solution
that brings testing out of the lab and to the point of care. The technology
provides healthcare professionals with an entire clinical lab in the palm of
their hand and is intended for use in non-laboratory settings, such as
physicians’ offices, urgent care clinics, elder care/assisted living
facilities, cancer treatment and dialysis centers, and potentially the
workplace.
Talis Biomedical Corporation,
a company dedicated to developing high-performance point-of-care diagnostic
tests for infectious diseases, today announced that it has secured a $25
million contract from the National Institutes of Health (NIH) for Phase 2
of its Rapid Acceleration of Diagnostics (RADx) initiative. Additionally, the
company has completed an additional financing of $100 million to scale
manufacturing for the launch of the Talis One™ diagnostic platform, which
provides rapid and highly accurate detection of COVID-19 at the
point-of-care.
Accurate Point of Care COVID-19 Testing
Today, patients are
often forced to endure days of waiting for lab results. As a result, doctors may initiate unnecessary
isolation or empirical treatment based on incomplete information. This approach
is costly and can be harmful to patients. With COVID-19 cases
on the rise in many areas across the country, there is a tremendous need for
access to testing, especially in vulnerable populations where the spread of
COVID-19 infection can be devastating. The Talis One System is a molecular diagnostic
platform developed to enable rapid, highly accurate point-of-care testing for
infectious diseases such as COVID-19.
Talis One COVID-19 Assays
The Talis One assays are based on a proprietary, highly
optimized nucleic acid isothermal amplification chemistry to achieve
exceptional test performance much faster than traditional PCR. The system is
designed for use in non-laboratory settings, such as physicians’ offices,
urgent care clinics, elder care/assisted living facilities, cancer treatment
and dialysis centers, and potentially the workplace.
COVID-19 is the first infectious disease that the Talis One
System will support. Future infectious disease indications may include assays
for other respiratory infections, such as influenza, as well as sexually
transmitted infections (STIs) and other infections impacting women’s health.
The Talis One instrument can be managed remotely and sends test results to a
cloud database for secure transmission, storage and review. Results are
available in 30 minutes or less.
“We are extremely proud that the NIH selected Talis,
out of a field of 600 applicants, to be among the first teams to move to the
final phase of the RADx initiative. This important funding will accelerate the
commercialization of our Talis One System for the detection of COVID-19.
Additionally, we are pleased to have the continued support of our investors,
who share our excitement about the significant impact the Talis One System can
deliver as a fast and reliable diagnostic testing platform for life-threatening
and life-altering infectious diseases,” said Brian Coe, Co-Founder and
Chief Executive Officer of Talis.
“In response to the significant need for rapid, highly
accurate testing solutions to help combat the pandemic, we were able to utilize
our Talis One System, which we have been developing for clinical use in women’s
health, to quickly develop an assay for SARS-CoV-2, the novel coronavirus that
causes COVID-19. We are particularly focused on serving vulnerable populations,
such as those in elder care facilities or patients with impaired immune
systems. With the support of the NIH RADx program and proceeds from the new
financing, we are confident that we will be able to accelerate our goal of
making rapid diagnostic testing widely available,” added Coe.
NIH Support
The Talis One COVID-19 assay project is supported by the
RADx program and has been funded in whole or in part with Federal funds from
the National Institute of Biomedical Imaging and Bioengineering, National
Institutes of Health, Department of Health and Human Services, under Contract
No. 75N92020C00010.