An overview of various aspects of the supply chain that would be crucial in the production and distribution of COVID-19 Vaccine. Write to us at connect@pharmashots.com if you want a PDF copy of this report.
Over the past seven months, the global pandemic caused by the spread of SARS-CoV-2, the virus that leads to coronavirus disease (COVID-19), has impacted the global economy and people’s lives tremendously. A number of countries and companies have concentrated their resources to produce a vaccine for COVID-19 and considering the present scenario, one can be hopeful to have a vaccine available soon. However, finding the vaccine is just the first step. Other critical steps that every government should be thinking about in relation to their national vaccine strategy are product integrity and safety, public education, and the actual distribution program itself.
Product integrity and safety tests that assure the vaccine is safe to use, communication programs that inspire public confidence, and a robust supply chain management strategy that anticipates local demand efficiently which will deliver a vaccination program that the general population is willing to participate in. Since the sense of urgency is critical, governments all over the world should start planning based on these important enablers. Without this planning, the goal of having a confident and vaccinated population ready to return to “normality” and participating fully in the economy will not become a reality.
On Tuesday 11th of August, Vladimir Putin announced the first vaccine to the world, named Sputnik V. With this announcement the race to get a working vaccine to people all over the world has heated up further between key players all over the world eager to cash in on this opportunity for financial and political reasons. One thing is clear, whichever vaccine makes it to commercialization the supply chain will play an integral part in the upstream manufacturing supply chain and the downstream distribution.
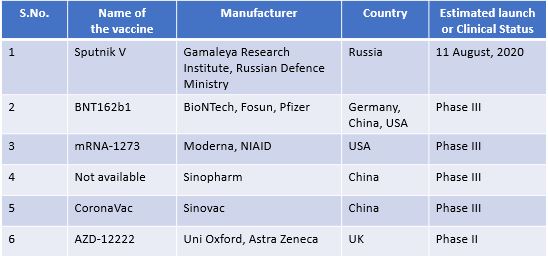
Some of the key contenders for the Covid-19 vaccine are:
Demand for the Vaccine
Many experts around the world are expecting the introduction of these vaccines to cause major challenges to global supply chains in manufacturing, transportation, and distribution as companies scramble to meet demand from countries all over the world. The demand for this vaccine will be at a historic high. Never in the world before has there been a situation where a vaccine needs to go out to the whole world at the same time. With a global population of more than 8 billion people, this will be more than a mammoth undertaking.
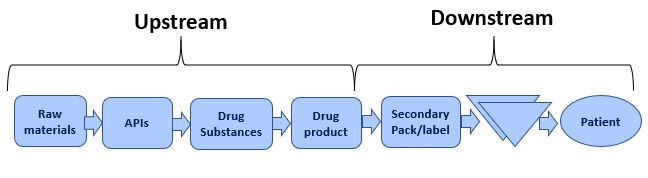
Upstream Manufacturing Supply Chain
To get to making a vaccine, there are several manufacturing steps that need to be taken which although invisible to most people are critical in the time path to a final drug product. These include making and testing the API product which forms the active ingredient of the drug. Making the actual drug itself (drug substance), the filling process involved in turning the bulk drug substance into usable vials or syringes for vaccination. The other needed parts related to the syringe itself, the needle, the vial, the cap, the label, etc. The last step is the actual final labeling and packaging of the vials into the finished product. In the pharmaceutical industry, all these manufacturing steps are usually performed at different factories often across different countries in the world. Synchronizing these production steps and the availability of all the components, the ingredients, and manufacturing capacity, as well as the logistics between the various factories all, create potential bottlenecks and risks.
Procurement of Vaccines – Access Rights

Governments around the world are competing to get first access to available vaccines. They are faced with critical questions around which vaccine brand to back now to secure access to large quantities to be able to start domestic immunization programs. Backing a vaccine and paying upfront for access and guaranteeing certain quantities of doses whilst there is no advance guarantee the vaccine will work is a large financial and political risk only the richer countries are able to make. The US, clusters of countries in Europe, and some other richer nations are making these types of advanced access agreements with key manufacturers. These funds help finance the manufacturing work already started whilst the vaccines are still being developed and tested for approval.
Although it is good that some of the richer nations have stepped up to help pre-finance these vaccine programs it presents a real moral dilemma once the vaccines are approved in terms of which countries get first access rights and which ones do not. Given the share volume of vaccines that need to be produced to cover the whole global population the difference between being a “first mover” country versus a “last mover” could be as long as 3-5 years.
In other words, countries with more money will buy priority access to vaccine programs whilst emerging countries will not be able to do so financially. It will mean that as the world moves out of the COVID-19 lockdown phase, clusters of countries can do so more easily than others. It will create a further disparity between regions around the world economically which potentially poses new socio-economic and (geo) political risks.
Downstream Supply Chain

Once a vaccine has been produced it is ready to be shipped under heavily regulated and temperature-controlled conditions to countries around the world. Many industry experts predict this enormous logistics task will lead to huge capacity problems for airlines and logistics companies. Laurent Foetisch, Supply Chain Management Consultant as Supply Chain Operations with more than 35 years of pharmaceutical manufacturing and logistics experience argues differently. “It is not possible to produce the quantities of vaccines needed all at once. Typically, vaccines are produced in batches of around 100,000 – 150’000 vials/syringes based on a daily production cycle. Even if multiple filling lines are used across various manufacturing locations it is not possible to produce all the vaccines immediately at these numbers in millions for everyone”.
This also means that the “big bang” launch theory of a vaccine might not be as big as many predict. It would mean that availability of vaccines for distribution is spread over a longer time frame which from a capacity perspective is less complicated to manage by logistics companies and airlines. Rather than a “bulk” shipment program, it could well turn more into a “Just in Time” shipment program feeding into domestic immunization programs.
However, there are other factors to think about as the first batches of vaccines are released and shipped. Eelco Dijkstra, Managing Partner at Europhia Consulting points out that ensuring product security across the entire supply chain will be vital. Not just in terms of establishing and using validated temperature-controlled transport and storage solutions but also in terms of risks such as the product being “highjacked” and/or “counterfeited” by organized crime as the product is transported across multiple countries.
National Vaccination Program – The Final Mile Distribution

The two major challenges of the government-run vaccination program on a national level would be in public education and in the distribution program itself. To make any vaccination program successful, it is vital to plan on how to educate the end community to ensure that the public is fully aware prepared to take the vaccination timely and efficiently.
Talking about the distribution strategy, the key questions that need to be addressed include how to best manage any vaccination program. The biggest first question for governments to address is whether a vaccine will be made mandatory for the whole population or be voluntary? Who will receive vaccination first? Vulnerable groups first such as people with a medical condition or the elderly? Will governments use hospitals throughout the country for people to come to and get vaccinated or will government look to use more points of vaccination such as medical clinics including private ones?
In some countries it will make sense to use existing COVID-19 infrastructure such as dedicated clinics and testing centers already in place. In some cases, it might make sense to set up “mobile” vaccine units which come to elderly homes, local community centres and schools.
Vaccines are pharmaceutical drugs and will need tight temperature and security control during storage and transportation. Given the share size of any vaccination program based on population size, it will run into the millions of vaccinations for which an end to end distribution model will need to be designed, tested, and executed in a very short period of time. Therefore, the timing for the planning for such a distribution model cannot start soon enough. For any vaccination program to be successful, the distribution part will be critical. Therefore, any national distribution plan linked to a country’s vaccination program will need to involve partnerships between government and private companies in logistics and distribution to plan and execute. We are already working with several governments on these initiatives providing input on strategic and tactical elements of such distribution plans.
Disclaimer: The opinions are based on the author’s own experience and understanding of the dynamics within the sector.
Europhia Consulting is an international management consulting company specialized in the logistics and supply chain industry in the life sciences sector. We operate global assignments for our clients.
Supply Chain Operations SA, based in Switzerland, is a specialized healthcare supply chain consultancy firm created in 2011 to serve the biopharmaceutical and Medtech industry.
We bring more than 120 years of end-to-end supply chain expertise to our valued customers. Both companies work together on strategic assignments for clients globally. We do not pretend to have been able to capture all challenges and all insights and have deliberately focused this strategy paper on some of the key challenges we see based on our work with clients within the industry.
For any questions or comments about this strategy paper please do not hesitate to reach out to us.

Eelco Dijkstra of Europhia Consulting has worked in supply chain and consultancy for over 25 years and in recent years has focused his expertise on the global pharmaceutical sector. He as worked a number of years for TNT Express and Kuehne & Nagel and in recent years managed his own consultancy practice.
Mobile: +31 624661688
Email: europhia@outlook.com
Website: www.europhia.com

Laurent Foetisch of Supply Chain Operations has extensive experience as a supply chain executive responsible for managing a global biopharmaceutical company in Switzerland for more than 20 years. In 2007 Laurent managed the supply chain integration between Merck and Serono with the responsibility to create one central supply chain structure using common processes and tools. Since 2011, Laurent runs its own supply chain boutique consulting firm named Supply Chain Operations SA.
Mobile: +41 792052332
Email: laurent.foetisch@supplychainoperations.ch
Website: www.supplychainoperations.ch