
PE firm Advent names its India API, CDMO platform as Cohance Lifesciences
The US-based PE firm which is betting on India pharma, said that it intends to add more assets in the future with the vision to […]

The US-based PE firm which is betting on India pharma, said that it intends to add more assets in the future with the vision to […]

The US-based PE firm which is betting on India pharma, said that it intends to add more assets in the future with the vision to […]

The US-based PE firm which is betting on India pharma, said that it intends to add more assets in the future with the vision to […]

The US-based PE firm which is betting on India pharma, said that it intends to add more assets in the future with the vision to […]

The US-based PE firm which is betting on India pharma, said that it intends to add more assets in the future with the vision to […]

The US-based PE firm which is betting on India pharma, said that it intends to add more assets in the future with the vision to […]

The US-based PE firm which is betting on India pharma, said that it intends to add more assets in the future with the vision to […]

CamPROBE may save vital time and money while reducing the risk of infection

ProteoNic’s protein expression technology improves recombinant protein production

For Medicare Advantage (MA) organizations, the stakes are high in today’s increasingly competitive environment, where savvy consumers who are dissatisfied with their plan have no […]

As a nation, let’s face our alcohol dependency head-on and have an honest discussion about the growing consumption trends.


Pfizer on Tuesday announced its maternal vaccine for RSV, administered during pregnancy, was effective at preventing infants from developing severe symptoms from birth through their […]

Shots: The P-III (MATISSE) trial evaluated the efficacy, safety, and immunogenicity of RSVpreF (120µg) vs PBO in a ratio (1:1) in 7400 patients aged ≤49yrs. […]

Patients 65 years or older, those with increased risk of cardiovascular disorders like heart attack or stroke, individuals who smoke or have for an extended […]
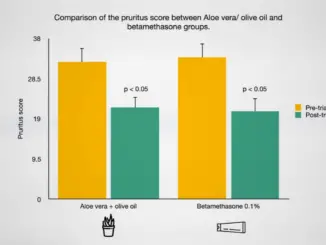
Psoriasis is a chronic, inflammatory skin disease that affects about one in 40 people, making it “one of the most frequent chronic skin diseases worldwide.” […]

In this episode, Alivia Leon, assistant editor, speaks with Christian Dunne, director of global corporate business development, Chargepoint Technology, about current and future processing equipment […]

Leaders at Veeva believe the wording around decentralized trials has been exaggerated, and that a bigger-picture outlook is important as the pandemic winds down.

The Bill & Melinda Gates Medical Research Institute’s clinical development leader talks about its new licensing deal with Merck.

Bristol Myers Squibb has announced positive topline results from its phase 3 trial of Reblozyl (luspatercept-aamt), the first erythroid maturation agent for first-line treatment of […]

Shots: MTx to receive an up front & research funding and is also eligible to receive clinical development and sales milestones along with royalties on […]

The US FDA provided 510(k) clearance for 23andMe to provide drug information for simvastatin based on the genetics of users of the service.

Altasciences is expanding its facility in Columbia, Missouri to provide bioanalytical services in support of preclinical and clinical studies conducted in the US.

The Association of Clinical Research Organizations (ACRO) has released a question and answer document to support the adoption of decentralized clinical trials (DCT).

Pfizer has bolstered the evidence behind its respiratory syncytial virus (RSV) vaccine with a positive trial of the shot used to protect newborns adding to […]

Shots: The US FDA has granted the FTD to DYNE-251 for DMD mutations amenable to exon 51 skipping. DYNE-251 is being studied in the P-I/II […]

Shots: The US FDA has granted the FTD to DYNE-251 for DMD mutations amenable to exon 51 skipping. DYNE-251 is being studied in the P-I/II […]

Nicox has had both good and bad news from a much-anticipated phase 3 trial of its glaucoma therapy candidate NCX 470. On the plus side […]

by MATTHEW HOLT I met Sarah MacDonald in the early 2000s. She is the ultimate extrovert who sings, cooks, maintains a huge circle of friends, […]

Human Immunology Bioscience aims to stand apart in autoimmune disease drug research with therapies that address cells at the root of a range of allergic […]

Did you know that it is possible for a surgeon to conduct a brain tumor surgery while you are awake without causing any pain? This […]

Brain surgery can take a toll on your body and recovering from it can be difficult for you. You should keep in mind that every […]

Eli Lilly’s links to Purdue University go back a long way, and have been reinvigorated with $92.5 million in additional funding and renewed research collaborations. […]

Shots: Eucure Biopharma to receive an up front & is also eligible to receive milestones along with royalties The collaboration enables ISU ABXIS to use […]

GP partners off work with burnout are being forced to consider returning to their job before they feel ready because of a yawning gap between […]

Surgery is one of the approaches used to tackle brain tumors. The term “surgery” may make you anxious and worried, but the procedure is safe […]

It took nearly a year for Kelly Macauley to realize the health plan she bought while shopping for insurance coverage last October was not, in […]

ST. LOUIS — It was not shocking that people listening to musicians covering Grateful Dead and Phish songs in October at a dive bar here […]

The federal government has eased its annual punishments for hospitals with higher-than-expected readmission rates in an acknowledgment of the upheaval the covid-19 pandemic has caused, […]

After you have received a diagnosis of a brain tumor, the next course of action would be to find the most suitable treatment for yourself. […]

Brain tumors are cancerous or non-cancerous growth of cells in the brain. Early diagnosis and timely intervention can help in tackling brain tumors. There are […]

In January 2020, the financial conglomerate Visa announced it was acquiring a relatively unknown startup, Plaid, for $5.3 billion. Corporate acquisitions like these are not […]

Shots: The US FDA has cleared an IND application to initiate the P-I (ERASER) trial evaluating the safety, PK & preliminary efficacy of ATG-017 + […]

Shots: The US FDA has cleared an IND application to initiate the P-I (ERASER) trial evaluating the safety, PK & preliminary efficacy of ATG-017 + […]

New research from the CPHI Annual Report 2022 – launched during CPHI Frankfurt (1-3 November, 2022) – predicts that huge volumes of Venture Capital (VC) […]

Shots: The US FDA has accepted the NDA of TransCon PTH (palopegteriparatide) for Priority Review in adult patients with hypoparathyroidism. The PDUFA date is expected […]

Shots: The US FDA has accepted the NDA of TransCon PTH (palopegteriparatide) for Priority Review in adult patients with hypoparathyroidism. The PDUFA date is expected […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

Glycopyrrolate injection is also indicated for use in adults as adjunctive therapy for the treatment of peptic ulcer when rapid anticholinergic effect is desired or […]

…well, at least fewer people are dying from natural disasters. This is from a recent New York Times article.

[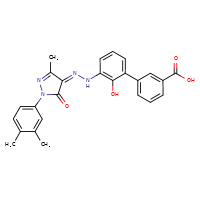](//www.DrugPatentWatch.com/p/preview/generic-api/eltrombopag?utm_medium=dpw_wp_blog&utm_campaign=dpw_wp_blog&utm_source=dpw_wp_blog) Eltrombopag is the generic ingredient in two branded drugs marketed by Novartis and is included in two NDAs. There are eight patents protecting […]

Annual Drug Patent Expirations for OXBRYTA Oxbryta is a drug marketed by Global Blood Theraps and is included in two NDAs. There are nine patents […]

[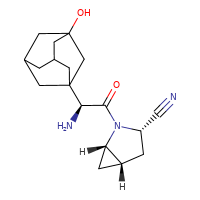](//www.DrugPatentWatch.com/p/preview/generic-api/saxagliptin?utm_medium=dpw_wp_blog&utm_campaign=dpw_wp_blog&utm_source=dpw_wp_blog) Saxagliptin is the generic ingredient in one branded drug marketed by Astrazeneca Ab and is included in one NDA. There are two patents […]

This chart shows the pharmaceutical companies with the most patents in Malaysia. Patents must be filed in each country (or, in some cases regional patent […]

CPHI Frankfurt is a hybrid event discussing emerging trends and novel technologies in the pharmaceutical industry.

Alcyr Araujo, CEO and founder of Mosyle It’s no secret providers are increasingly reliant on mobile devices like cell phones, tablets, and laptops to improve […]

The post Trust is essential to a functioning social safety net: Lessons from the pandemic appeared first on Healthy Debate.

[Federal Register Volume 87, (Tuesday, November 1, 2022 )] [Notice] [Pages 65828-65829] National Advisory Committee on Occupational Safety and Health (NACOSH): Notice of Meetings


Open Enrollment starts today! You have until January 15, 2023 to apply for new health coverage or change your health plan for 2023. If you […]
Copyright © 2024 | WordPress Theme by MH Themes