Over time, several innovative technologies have been developed to support the designing and manufacturing of various types of antibody products; however, the aforementioned processes are characterized by high costs and technical complexities, especially during the production and downstream processing steps. Therefore, it is challenging for new entrants and companies with limited resources and capital, to sustain their respective businesses, without additional support, in this market. In order to help antibody developers address their product development and further processing-related concerns, and keep pace with the evolving needs of consumers of advanced therapy medicinal products, there are a number of companies that offer services related to the development, production, and downstream processing of antibodies. Presently, outsourcing antibody purification operations has become a popular practice, even among sponsor companies that their proprietary production expertise and associated infrastructure,
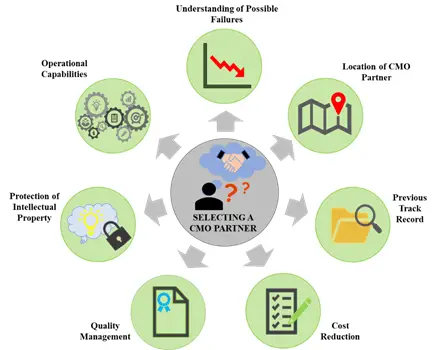
The process of antibody purification is complex and selecting a suitable service provider for outsourcing is one of the main challenges faced by companies engaged in antibody development and manufacturing. Despite the cost benefits they offer and their extensive technical capabilities, there are a number of parameters that need to be considered while selecting a suitable service partner. The selection of an inappropriate service provider partner can prove to be disastrous in the long run, when issues, such as delays and cost overruns, crop up.
The evolving landscape of antibody purification service providers
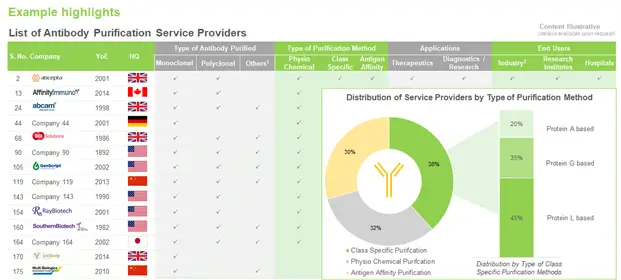
During our research, we identified over 150 companies that claim to offer antibody purification services. Although, players involved in the purification of antibodies are well-distributed across different regions of the globe, however, the majority of them (nearly 50%) are based in North America.
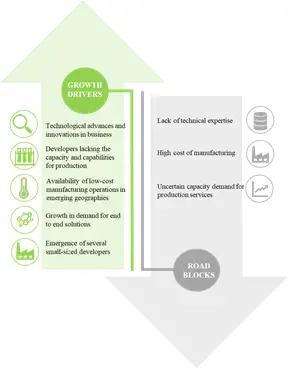
Despite the increasing opportunity in this domain, high entry costs (associated with building the required technical expertise and setting up of facilities) and rising regulatory stringencies, are the primary impediments to the number of stakeholders entering this market. For sponsors looking to outsource, there are multiple areas of concern associated with engaging service provider entities; some of the prominent challenges faced include, shortage of purification capacity, and process transfer related complexities. Therefore, it is imperative for stakeholders in the services segment of the industry to be address existing areas of concern to ensure optimal market growth over the next decade.
Outsourcing allows companies in this domain to focus on their core competencies and also enables significant cost saving opportunities. Moreover, engaging a service provider has also been demonstrated to improve operational efficiencies, and expedite time-to-market. Most modern pharmaceutical service providers also offer regulatory affairs management support. The demand for companies offering antibody purification services has increased over the past several years and we believe that this trend is likely to persist in the future as well.
To get a detailed information on the key players, recent developments, and the likely market evolution, visit this link
 Global Antibody Purification Services Market, 2021-2030
Global Antibody Purification Services Market, 2021-2030
You may also be interested in the following titles:
- HUMIRA® (Adalimumab) Biosimilars: Focus on Approved & Launched Biosimilars, Investigational & Research Use Biosimilars, Inactive / Terminated / Withdrawn Biosimilars, Industry / Non-Industry Partnerships
- CRISPR Based Therapeutics Market, 2021-2030
- Display Library Technologies and Affiliated Services Market, 2021-2030
The post Antibody Purification: A Deeper Look into Service Providers Landscape appeared first on Blog.
