Over the last decade, autoinjectors have emerged as a promising therapeutic commodity for safe administration of parenteral therapies. Patients who suffer from chronic diseases, such as diabetes, rheumatoid arthritis and multiple sclerosis, often require frequent dosing of medication which poses significant inconvenience to them for visiting proper healthcare centers. Hence, autoinjector devices enable such patients to achieve the desired medication adherence at the comfort and safety of their home.

Moreover, the integrated safety mechanisms and hidden needles, help in reducing risk of needlestick injuries and dosage errors (which may be triggered by exposed needles and syringes). In conclusion, the autoinjector devices aid in improving the quality of life and provide cost benefits.
Several industry players are exploring the use of autoinjectors for a variety of therapeutic indications. For instance, the recent autoinjector combination device approvals, such as AJOVY® (Teva Pharmaceutical Industries), Fasenra® (AstraZeneca), NUCALA Autoinjector (GlaxoSmithKline), Vyleesi Autoinjector (AMAG Pharmaceuticals® / Palatin Technologies) and Teribone
Autoinjector (AMAG Pharmaceuticals® / Palatin Technologies) and Teribone (Asahi Kasei Pharma), have been introduced for a variety of indications, including migraine, asthma, hypoactive sexual desire disorder (in women) and osteoporosis.
(Asahi Kasei Pharma), have been introduced for a variety of indications, including migraine, asthma, hypoactive sexual desire disorder (in women) and osteoporosis.
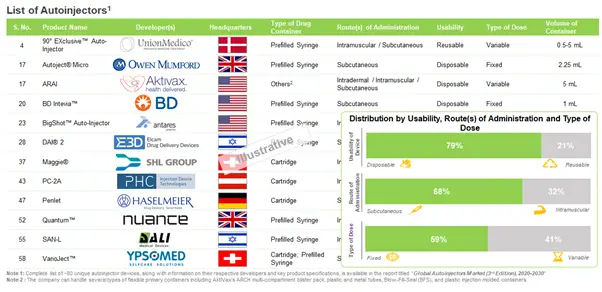
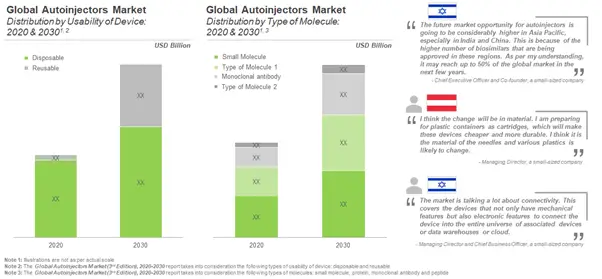
Industry Landscape Suggests that Most of the Autoinjector Device Developers Manufacture Disposable Autoinjectors
During our research, we came across about 80 autoinjectors that are available / being developed by over 20 players. Most of the devices listed can be customized for the administration of therapeutic products with varying viscosities and according to the nature of drug molecule. The devices can be differentiated on the basis of several parameters, such as usability, type of primary container, volume of container, type of dose, route(s) of administration, actuation mechanism and feedback mechanism.
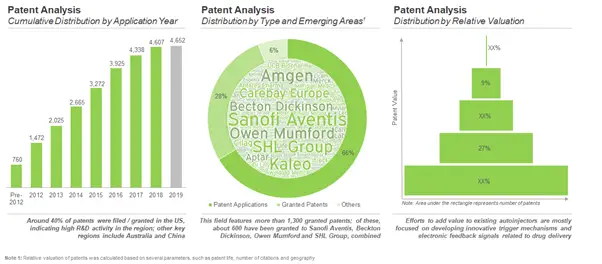
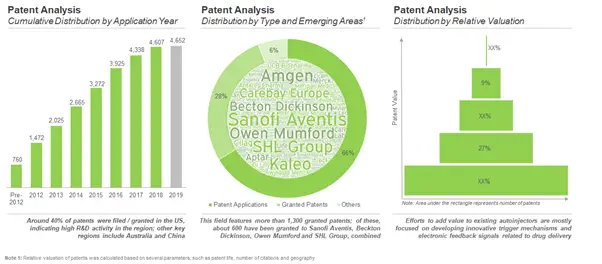
Intellectual Property Landscape looks Promising with over 4,600 patents application been filed / granted
Lately, stakeholders in the medical devices industry have been making significant investments towards the development of next generation autoinjectors with innovative features and enhanced capabilities. The surge in approval of biologics for delivery via parenteral routes has led to an increasing demand for autoinjectors that enable sustained release of viscous formulations of the biologic drug. Given the ongoing efforts to design improved versions of autoinjector devices, it is important for innovator companies to also develop meaningful intellectual property patenting strategies. Over time, more than 4,600 patents have been filed / granted related to autoinjectors and affiliated products.
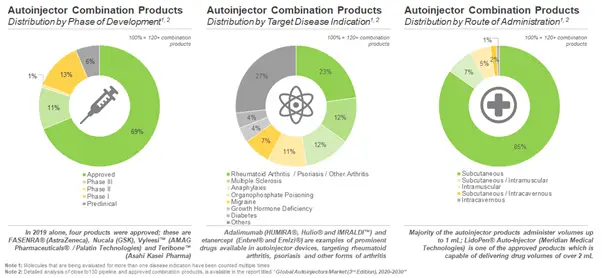
More than 70 Companies are Engaged in Introducing Autoinjector Combination Products in the Market
Current landscape suggests there are over 90 drug-device combination products available in the market. The autoinjector combination products can be differentiated on the basis of their parameters, such as the initial year of approval of the combination product, usability, route of administration, drug class, type of molecule, dose strength, therapeutic area and other packaging formats of the approved therapeutics.
Autoinjector Devices Market to Grow at a Healthy Rate in the Foreseen Future
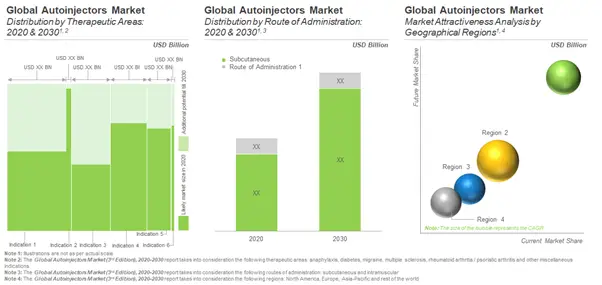
The rising need for patient-friendly and safe drug delivery systems is expected to sustain the growth opportunities in this market in the mid to long term. According to our estimates, the autoinjectors market is anticipated to grow at an annualized rate of around 10%. The current market is driven, to a large extent, by the sales of autoinjectors designed to deliver drugs for the treatment of rheumatoid arthritis and diabetes. However, devices indicated for treating other clinical conditions, such as asthma, hypercholesterolemia, acute repetitive seizures, Crohn’s disease and ulcerative colitis, which require frequent / emergency dosing are expected to drive growth in the long term.
According to our estimates, North America (primarily the US) and Europe are expected to capture the largest share (about 70%) of the autoinjectors market in 2030. This is likely to be followed by Asia-Pacific and rest of the world.
To get a detailed information on the key players, key opinion leaders and likely drug candidates for autoinjector based administration, drug delivery device contract manufacturers and other key industry trends impacting this domain and the likely market evolution, visit this link
Global Autoinjectors Market (3rd Edition)
You may also be interested in the following titles:
- Large Volume Wearable Injectors Market (5th Edition), 2020-2030: Focus on Bolus, Basal and Continuous Delivery Devices
- Prefilled Syringes Market (5th Edition), 2020-2030
- Microneedles and Needle-Free Injection Systems / Jet Injectors (Devices based on Spring, Gas and Other Mechanisms) Market, 2019-2030 [COVID-19 Series]
The post Increasing Prevalence of Chronic Diseases- Driving the Demand for Self-Administration Devices, Like Autoinjectors appeared first on Blog.
