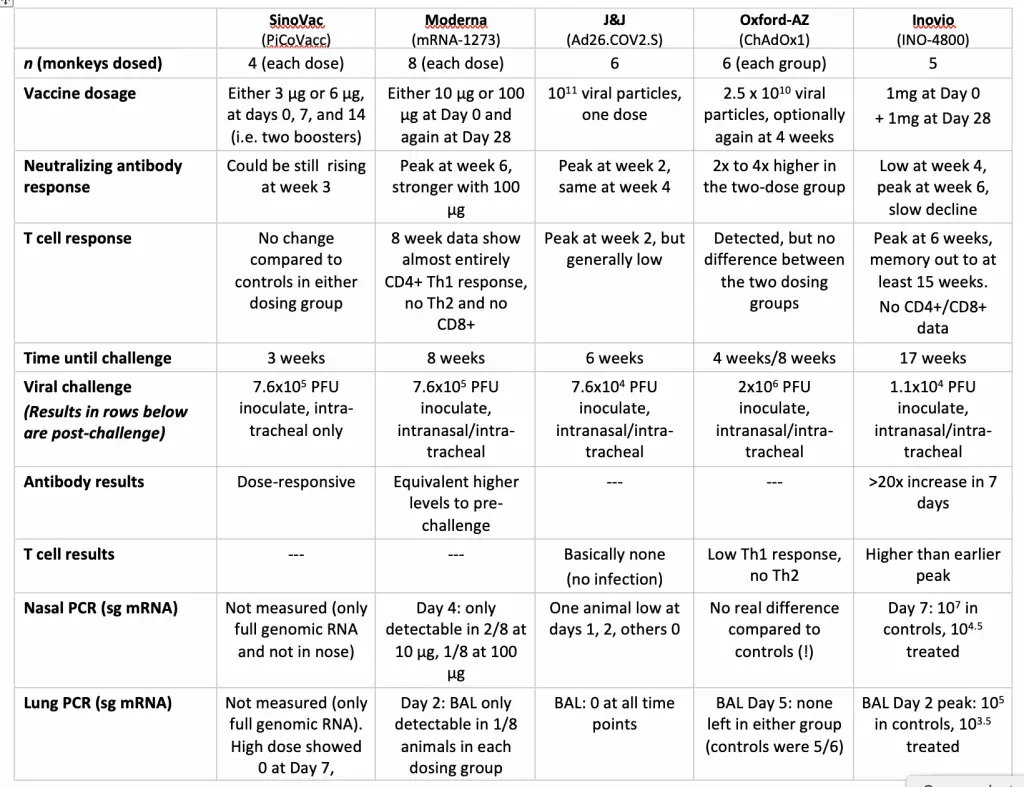
We have a sudden influx over the last few days of preclinical rhesus challenge studies with various coronavirus vaccines, and it’s only natural to try to compare them. I have worked up a table with all four of the current results and the previously reported SinoVac inactivated virus vaccine, whose rhesus challenge numbers were officially published earlier this month. This is the closest to Phase II human data that we’re going to have until later in the summer, so let’s see what we can make of it!
Here is the Oxford/AstraZeneca vaccine, ChAdOx1, which is (as many have had the chance to memorize by now) a chimpanzee adenovirus repurposed with genetic material from the coronavirus Spike protein. This is the final published version of a preprint that came out earlier this year, whose results attracted wide comment and some criticism at the time. Since this one has already been discussed here, I’ll defer it to the comparison table I’m putting together.
And here we have Moderna’s mRNA-1273 results. The short readout is that the vaccine induced an antibody response in the monkeys similar to that seen in convalescent human serum, and a T-cell response that was heavily biased towards CD4+ Th1, with no detectable CD8+. That’s quite similar to what the company has reported in the human Phase I trials, which may add to one’s confidence in trying to learn something from the primate viral challenge studies. We’ll go into the actual numbers in the comparison table, but although the challenge in this case showed strong effects, it was not quite “sterilizing immunity” where the possibility of viral infection is completely shut down.
Then comes J&J, and I believe that these are the first numbers from their vaccine, which is another adenovirus vector, but an obscure human strain (Ad26) rather than a chimpanzee one. It’s been a bigger effort than we knew: the paper shows that they investigated seven different forms of an Ad26 vaccine, coding in various amino acid mutations for stability, different “leader sequences” to try to get the genetic material expressed more robustly once into cells, and different antigen sequences entirely. All of the latter are variations on the Spike protein, but some are full-length, while some had the cytoplasmic “tail” region or the transmembrane region deleted. They also tried mutations in the furin protease cleavage site and (like the first now-dropped Pfizer mRNA candidate), tried a foldon trimerization domain to present the antigen in multiple ways.
The one that stood out in antibody response was the “S.PP” candidate: wild-type leader sequence, full-length Spike protein, furin cleavage site mutated, and proline stabilizing mutations, so that’s what will be in the comparison below. Interestingly, this candidate had the lowest T-cell response of all seven that they tried (their paper’s Figure 3). After looking over the challenge data, the paper has some conclusions (their Extended Figure 6) on immune correlates (what markers are most indicative of efficacy): “these findings suggest that serum antibody titers may prove a useful immune correlate of protection for SARS-CoV vaccines. By contrast, vaccine-elicited ELISPOT responses, CD4+ ICS responses, and CD8+ ICS responses did not correlate with protection“. Which is interesting!
And finally, we have the Inovio DNA vaccine (INO-4800), which I’m glad to finally be seeing more data on. This is a DNA construct for the Spike protein, delivered intradermally. We need various technologies and routes of delivery to be running simultaneously, so the publication of this preprint is very timely. INO-4800 also produced antibody and T-cell responses, which will be summarized below.
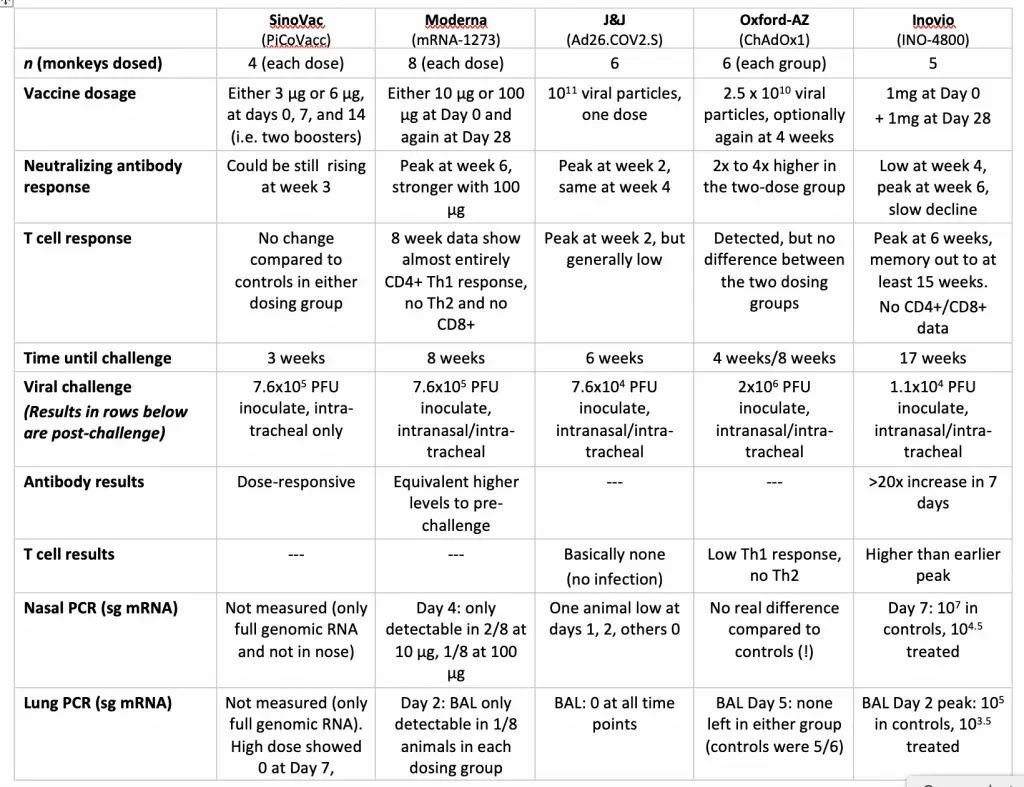
There are a number of take-aways from this. I particular want to draw attention to the “Virus Challenge” row, since not all of these studies have been performed with an equivalent amount of virus. I have taken the various measurements as given in the papers and converted them all to Plaque Forming Units, using the conversion factor between that and the TDIC50 measurement given in the Moderna paper. You will note that Inovio’s challenge is the weakest of all those in the table, but at the same time their response is also the weakest: none of their animals ever go down to zero when measuring subgenomic RNA (which is more indicative of replicating virus). I am not optimistic, based on these numbers, about how their candidate will perform in Phase II/III human studies, both on the absolute scale and relative to their competition.
The second lowest is J&J. Their efficacy number are very good indeed, but one has to wonder if Moderna (whose viral challenge dose appears to have been tenfold higher) and the other competitors might have shown similar numbers at that same dose. On the other hand, that was only one dose of the J&J vaccine, which is impressive – they are the only company so far to report such a dosing protocol, and that’s a very big deal, from a logistics standpoint. But on the third hand, that single dose was tenfold more viral particles than the other adenovirus vector in the table (Oxford), so is J&J prepared to manufacture five times more active viral vector? They will, on the other hand, be using up only half as much fill-and-finish capacity after that. And so on – isn’t drug development a blast? Don’t you just wish that you could have decisions like this hanging over your head for a living?
I wish that we had similar primate challenge data for the Pfizer/BioNTech candidate, but I honestly don’t even know if they ran that experiment, or when we’ll see it. I still like that one from what we’ve seen of what we now know was its inferior competitor in Phase I, and I like the J&J vaccine as well. Those would be my personal front-runners, with Moderna and Oxford right behind them. Unknown factors in the human efficacy trials could rearrange that list in any direction, though, so my handicapping isn’t worth that much. That said, I don’t have similar hopes for the Inovio candidate based on what we’re seeing, and I don’t really know what to make of three-dose SinoVac inactivated-virus vaccine. Two boosters would be a major pain, and the data package on this one is, in retrospect, the thinnest of the bunch (although it does indeed appear to have worked). I’m not sure if anyone knows what they’re doing in their clinical trials.
On to Phase II!
