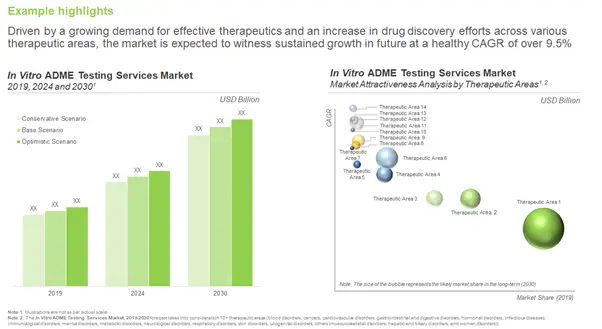
It is important to note that the process of drug discovery is extremely demanding, both in terms of capital and time. In fact, the overall amount spent on R&D initiatives in the pharmaceutical / biotechnology sector has increased from around USD 128 billion in 2008 to USD 165 billion in 2018. Moreover, only a small fraction of early stage therapeutic candidates are able to make it past preclinical evaluation. According to a study conducted on terminated drug development programs, the high rate drug failure in clinical trials was primarily attributed to problems associated with their pharmacokinetic profiles, absorption, distribution, metabolism and excretion (ADME) properties and inherent toxicity.
With increasing cases of drug failure, due to problems associated with pharmacokinetic profiles of candidate therapies, ADME properties and inherent toxicity, industry players are actively looking for more advanced solutions. Drug developers prefer to opt for contract service providers that offer a range of capabilities, such as design, synthesis, initial scale-up, in vitro ADME testing, safety pharmacology, under one roof; this guarantees a certain degree of ease of operation, and enables sponsors to shortlist and rely on a capable partner for their outsourcing requirements.

Service Providers Offering In Vitro ADME Testing Services
Over 95 CROs, featuring a mix of small-sized (less than 50 employees, 42%), mid-sized (50-200 employees, 17%), large companies (200-1,000 employees, 15%) and very large companies (more than 1,000 employees, 26%), offer contract services for in vitro ADME testing.
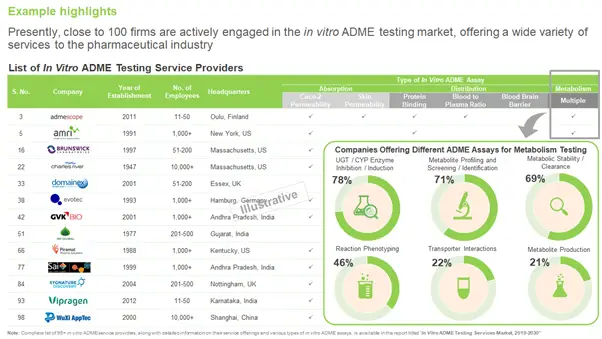
To know about the companies that claim to offer in vitro ADME testing services, check out our report here
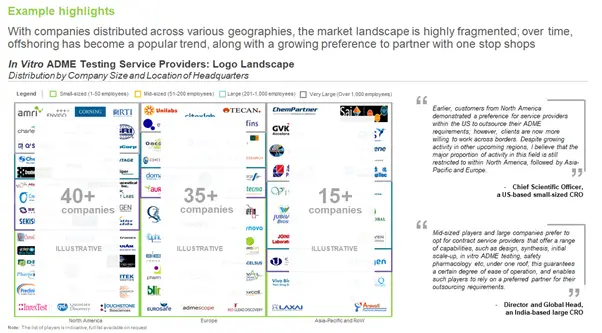
What is the Regional Distribution of Service Providers?
Majority of the CROs (nearly 80%) are based in North America and Europe. Within North America, the US has the maximum number of players, whereas, in Europe, most of the service providers are distributed across France, Germany, the UK, and Spain. A relatively smaller, but growing, proportion of such players are situated in the Asia-Pacific region; India, China, and Australia have a significant number of firms that claim to offer ADME testing services.
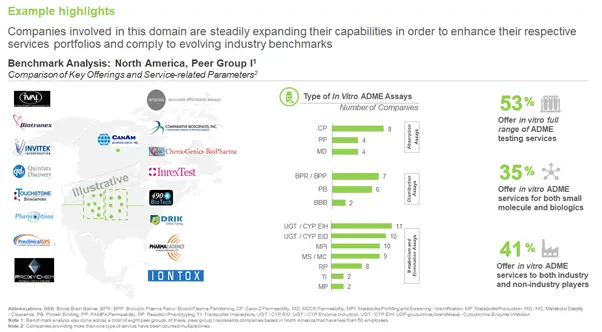
Which Company Has an Edge Over Other Companies?
With so many players in the market, it may become baffling to decide on which company would serve your purpose. Some companies do have advantages over other competitors, in terms of their experience, and service portfolio. With the intention to develop a better understanding of the overall potential and capabilities of industry players involved in this domain, we carried out a benchmarking analysis of the various stakeholders across different geographies.
Recent Advances
Recently, in February 2020, Eurofins Discovery and PharmaResources, a leading CRO based in China, announced a commercial cooperation agreement to provide PharmaResources’ customers with expedited access to Eurofins Discovery’s pharmacology and ADMET portfolio to accelerate their client’s drug discovery timelines. Earlier in July 2019, SEKISUI XenoTech announced collaboration with the Drug Development Solutions Center to offer a full suite of ADME testing services. In May 2020, SEKISUI XenoTech received a US patent for an in vitro method to evaluate xenobiotics as immune-modulators of drug transport and metabolism For More Insights check out the report here
In Vitro ADME Testing Services Market, 2019-2030
You may also be interested in the following titles:
- Microneedles and Needle-Free Injection Systems / Jet Injectors (Devices based on Spring, Gas and Other Mechanisms) Market, 2019-2030 [COVID-19 Series]
- Global Autoinjectors Market (3rd Edition), 2020-2030
- Prefilled Syringes Market (5th Edition), 2020-2030
The post Increasing Rate of Drug Failure has Prompted the Drug Developers to Rely on CROs offering In Vitro ADME Testing Services for their Outsourcing Requirements appeared first on Blog.
