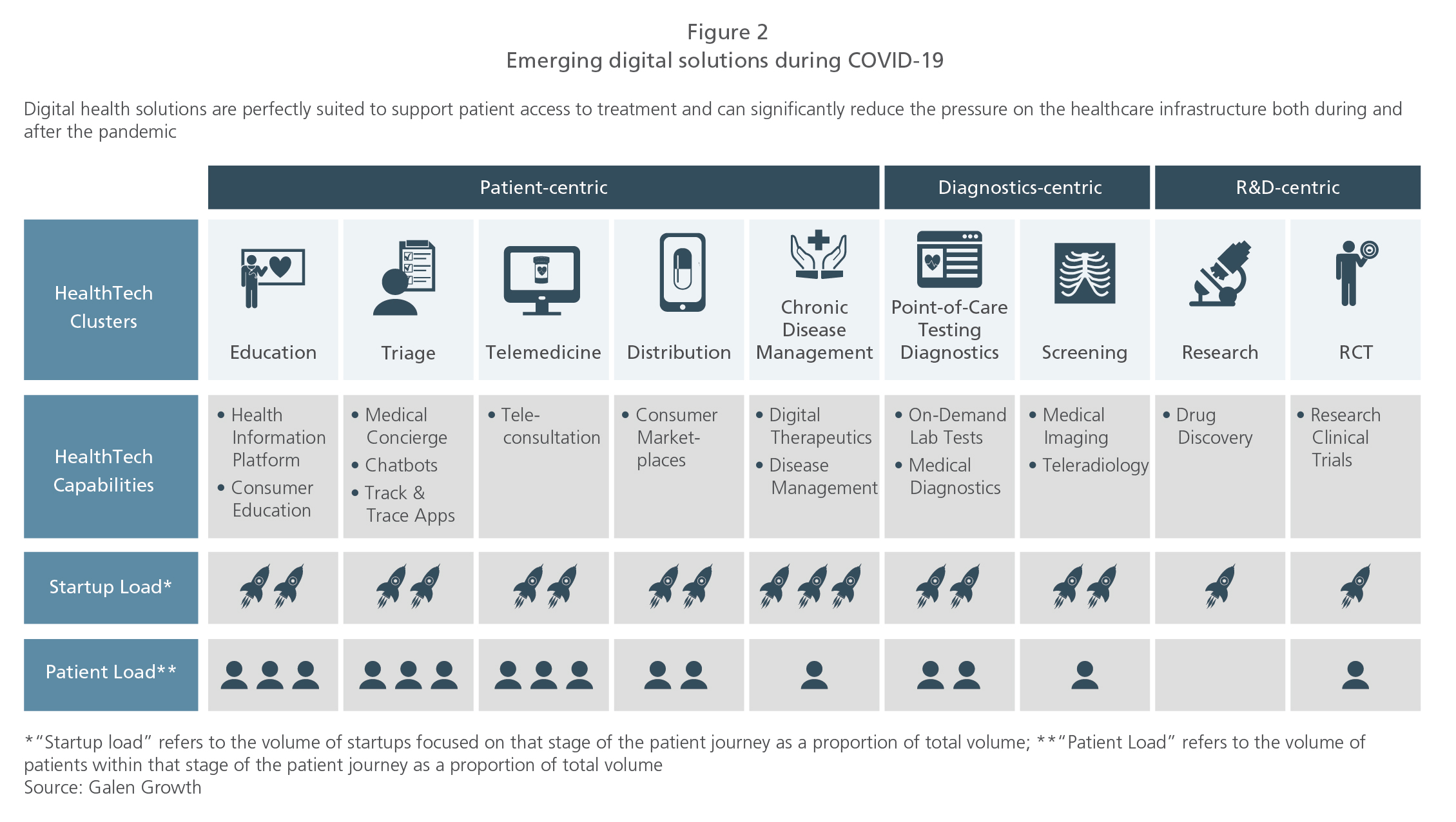
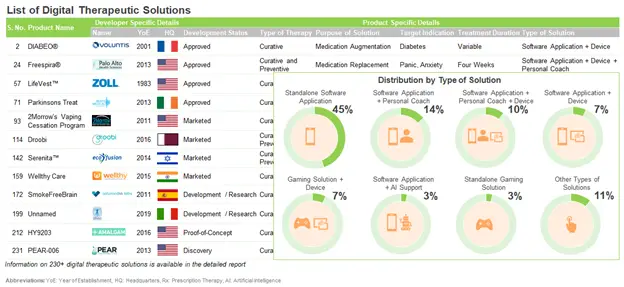
Digital therapeutics are clinically validated applications / software / online programs that have demonstrated the capability to facilitate positive outcomes when used in the prevention / treatment / management of diseases / clinical conditions. These therapeutics are designed to engage patients in personalized treatment or disease prevention programs, through mediating behavioral or psychological modifications, providing motivational support and inculcating healthy lifestyle changes. Presently, several companies are focused on the development of prescription digital therapeutic solutions.
What is Current Developmental Scenario Across the Globe?
There has been a significant increase in the number of companies established during 2016-2017. This can be attributed to the fact that more than 80% digital therapeutic have been approved since 2015. Moreover, formation of Digital Therapeutics Alliance in 2017 further spurred in the interest of stakeholders in this domain. In addition, nearly 85% companies have been established post 2011, which highlights the growing interest within this domain.
Even though efforts for development of digital therapeutic solutions have been undertaken by players based all across the globe, majority of the developers (68%) are headquartered in North America. It is worth mentioning that within North America, maximum number of players are headquartered in the US (93%). Similarly, within Europe, more than 60% players are based in UK, France, Germany and Switzerland. Further, Australia, Israel, Japan and South Korea are major hubs in Asia-Pacific region.
Key Initiatives by Regulatory Authorities to Expand Digital Therapeutics in COVID-19
The current crisis has created a heavy demand for the remote patient monitoring and treatment (primarily due to the fact that patients cannot physically meet the healthcare providers due to COVID-19 restraining orders). In this situation, the importance of digital therapeutic solutions was realized by healthcare professionals to easily track / monitor the patient progress and provide appropriate measures remotely. Further, the USFDA has taken initiative to provide approval under the category of emergency use authorization (EUAs) for many such remote monitoring systems and technologies that operate along with wearable devices. In addition, in June 2020, the USFDA issued enforcement policy to support the development of these solutions to cater to the growing demand and mitigate the risk of healthcare personnel being exposed to the disease.
Bottom Line: The Demand for Digital Therapeutics is Skyrocketing
Given the current situation, it is safe to assume that a significant proportion of the healthcare budget is being allocated to develop and distribute digital health solutions, including digital therapeutics and monitoring solutions. As a result, the demand for such solutions may be anticipated to continue to grow in the immediate future.
Check out our new Reports Here-
Roots Analysis Report insights
You may also be interested in the following titles:
- STING Pathway Targeting Therapeutics and Technologies
- Point-of-Care Diagnostics Market for Infectious Diseases by Indication
- Single-cell Sequencing Services and Technologies Market, 2020-2030
The post Digital Therapeutics: Key Concepts and Regulations to Tackle COVID-19 Pandemic appeared first on Blog.
