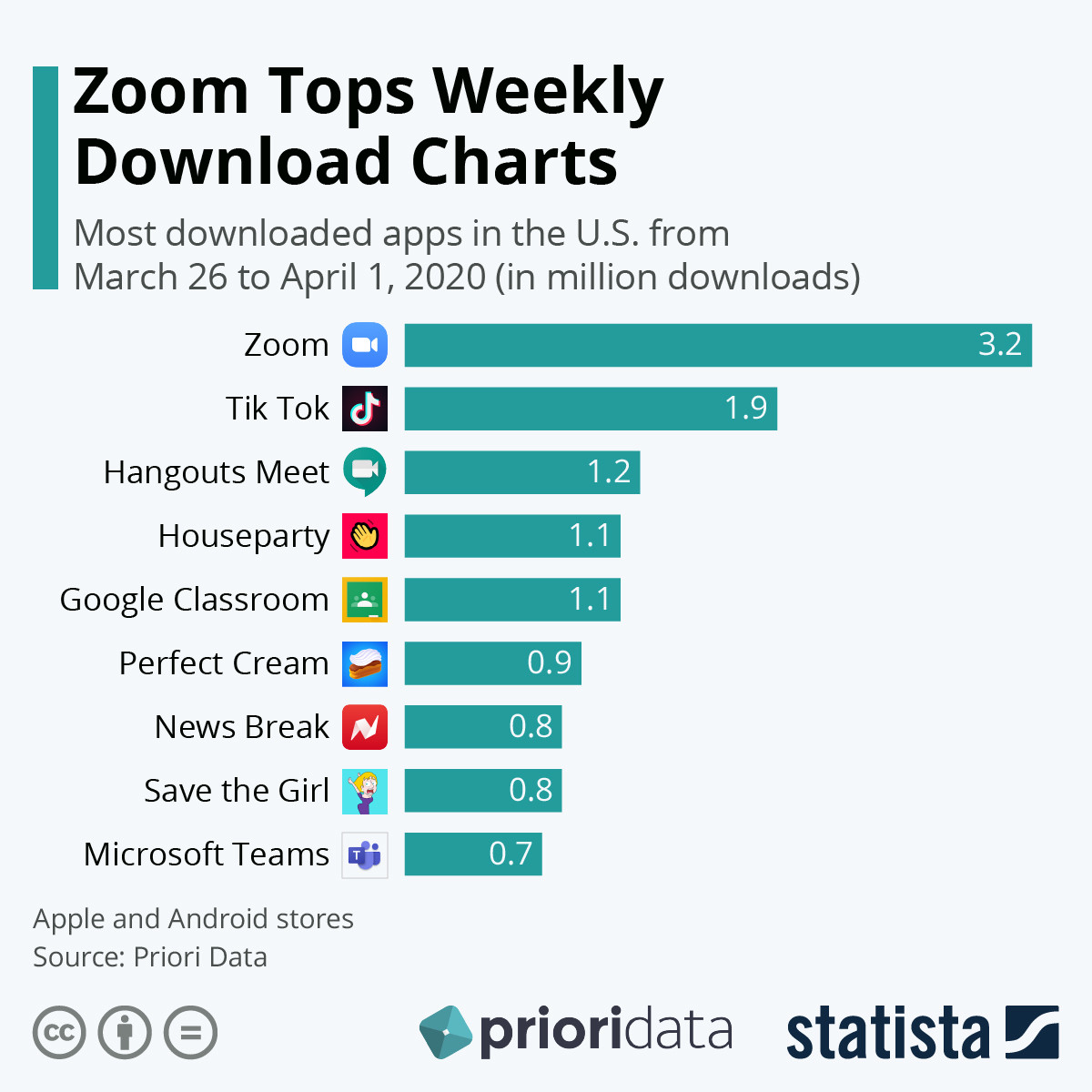
Since, the outbreak of novel coronavirus, drug repurposing has become one of the core research strategies for finding a treatment. Several clinical studies for the already approved drugs, have been registered in order to identify cure for the novel coronavirus infection. It is known that on an average, it takes around 12 to 15 years to develop and commercialize a novel drug product, involving high financial investments. On the other hand, drug repurposing has become an integral part of the pharma and the healthcare sector globally. This is due to the fact that drug repurposing offers costs and time related benefits in early stages of development. Presently, drug repurposing is one of the viable options for the pharma industry to win against the pandemic. This pandemic has led increased interest in this domain.
What is the role of drug repurposing service providers?
Many start-ups, mid-sized and well-established players are primarily focusing on collaborating together so as to minimize the workload on the big firms for the development and the commercialization of a drug in lesser time. Several number of service providers, including contract research organizations (CROs), claim to provide the necessary support to drug developers in identifying / predicting prospective drug candidates for repurposing. Such companies offer a variety of services, encompassing both drug discovery operations and consultancy requirements. In fact, many of such players claim to have developed proprietary platforms based on advanced technologies, such as big data analysis, artificial intelligence (AI) and real-world evidence, in order to facilitate drug repurposing related decision-making.
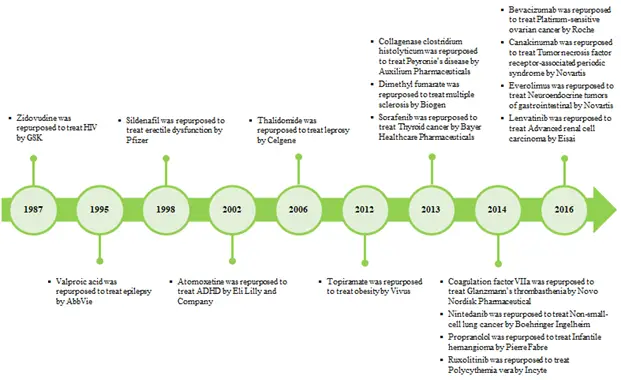
Since 2011, the drug repurposing domain has become an attractive alternative to the slow pace traditional drug discovery and development process. There are few notable examples that could quote the success of this methodology such as Pfizer’s Sildenafil and Grunenthal’s Thalidomide. Sildenafil was originally developed to treat coronary artery disease in 1980. But in the year 1998, it became a blockbuster due to its new use in treating erectile dysfunction.
What are the recent initiatives undertaken in the field of drug repurposing, to combat COVID-19?
In the past few months, several drug repurposing service providers have undertaken various collaborative initiatives, to develop vaccines / biologics as the potential therapeutic against COVID-19. For instance, in April 2020, SOM Biotech partnered with Ewha Womans University to identify targets against coronavirus by using SOM Biotech’s technology. Some of the prominent examples of approved drugs that are being evaluated to treat COVID- include chloroquine / hydroxychloroquine (anti-malarial drug) and remdesivir (initially developed to treat hepatitis C). Due to the rapid advances in the field of biology, genomics and bioinformatics, drug repurposing strategy has been actively engaged in finding ways for combating rare diseases in recent years. It is expected that the process of identifying new uses for existing drugs will continue to gain popularity in the near future as well. Thus, greatly helping the progress of the medical and the healthcare sector
Visit this link for more Insights.
Roots Analysis – Pharmaceutical, Biotechnology and Medical Devices.
You may also be interested in the following titles:
- China Biopharmaceutical Contract Manufacturing Market, 2020 – 2030
- Continuous Manufacturing Market (Small Molecules and Biologics), 2020 – 2030
- Prefilled Syringe Fill / Finish Services Market, 2020-2030
The post Will the Drug Repurposing Industry Boom due to the Pandemic? appeared first on Blog.
