cbaker_admin
Thu, 07/23/2020 – 15:30
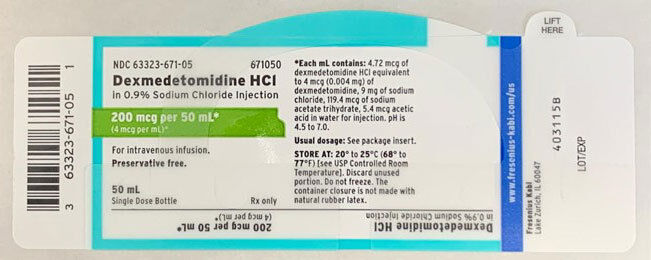
Fresenius Kabi USA announced a voluntary, nationwide recall of two lots of dexmedetomidine HCl in 0.9% sodium chloride injection, 200 mcg/50 mL (4 mcg/mL), 50 mL fill in a 50 mL vial due to the possibility of a trace amount of lidocaine in the lots. No adverse drug experience reports have been received for either lot thus far. Fresenius Kabi notes, “Administration of dexmedetomidine HCl containing trace amounts of lidocaine to a patient with lidocaine allergy, however, could result in anaphylaxis, a potentially life-threatening condition.” The affected lots were distributed nationwide to wholesalers, distributors, hospitals, and pharmacies between June 3, 2019 and April 8, 2020.
