Human Microbiome Therapies are Viable Alternatives to Antibiotics, with Substantial Therapeutic Potential
The term microorganisms was perceived to be synonymous to harmful, disease causing agents, until the discovery of certain beneficial microbial colonies in the human system. Currently, humans and a variety of species of bacteria, yeast and protozoa, are known to co-exist in mutually beneficial relationships. Moreover, it is estimated that the human microbiome consists of about 100 trillion microorganisms; it is interesting to note that this figure significantly outnumbers the total number of cells in the human body. Majority of the microorganisms benefit humans by supplementing them with traits that they would otherwise not possess. The healthy variety of bacteria in the gut has the significant potential to enhance the immunity and offer a wide range of health benefits. This has led to extensive R&D efforts by human microbiome-based therapy / diagnostic developers to make significant strides, in terms of progressing proprietary product candidates into the clinic. In addition, the existence of a strong link between the human microbiome and health, a myriad of therapeutic interventions based on manipulation of commensal microbes, are under development.
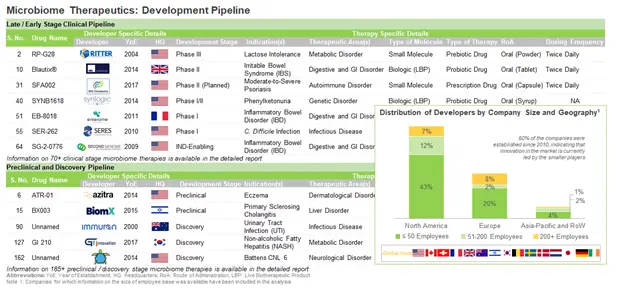
Microbiome Therapeutics- Current Market Landscape
Over the last few years, there has been a significant growth in interest in the field of microbiome therapeutics. During our research, we were able to identify 72 drugs that are under various stages of clinical development and 188 microbiome therapeutics which are either under preclinical or discovery stage of development, indicating that the microbiome market is still in its infancy.
During our research, we identified over 90 companies that are involved in the development of microbiome therapeutics. Of these, 67% of the players are very small / small-sized companies (less than 50 employees), 17% of the players are mid-sized companies (51-200 employees) and 16% are large players (more than 200 employees).
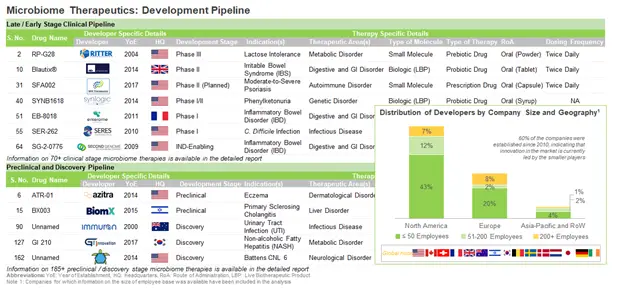
Over 60% of the companies are established in North America, majority (54) of which are based in the US. Within the US, California, Florida, Maryland, Massachusetts, New York, New Jersey, North Carolina, and Pennsylvania, have emerged as the most important regions where multiple microbiome developers are presently located.
Over 80% of the clinical stage microbiome therapeutics are biologics. It is worth noting that there are five biologics and one small molecule, which are currently in phase III of development. A similar trend was observed in case of the preclinical pipeline candidates. It can be observed that 83% of the early stage drugs are biologic in nature. This can be attributed to the fact that biologics have demonstrated more promising results in the early stages and are, therefore, progressing towards clinical stages of development. It is worth noting that live biotherapeutics (LBPs) emerged as the most preferred biologics within this domain.
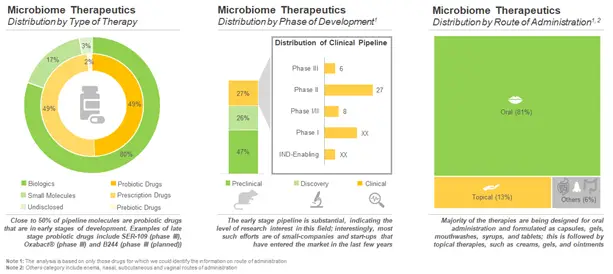
Moreover, majority of the clinical drugs (45) are designed for administration through oral route. In fact, the oral route is one of the most preferred routes of drug intake as it offers various benefits, such as high bioavailability and rapid delivery. In addition, there are seven drugs targeting dermatological disorders and infectious diseases which can be delivered through the topical route.
Microbiome Therapeutics have Demonstrated the Potential to Target a Wide Array of Disease Indications
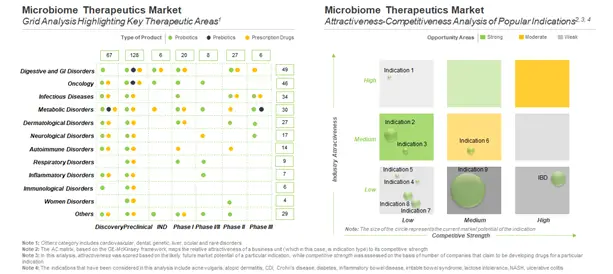
Microbiome therapies, once approved, are likely to change the current therapeutic landscape. These therapies are claimed to have the potential to provide a holistic treatment, due to which they are likely to have a reasonable impact on the market, specifically shifting the treatment modalities in disease areas, such as (in alphabetical order) digestive and GI disorders, infectious diseases, metabolic disorders and oncological indications.
Majority of the molecules in the microbiome pipeline target digestive and GI disorders, including IBD, IBS and AAD. Over the years, oncology has emerged as a popular area of interest to developers of microbiome therapeutics. Despite significant advancements in elucidating the role of the microbiome in the onset and progression of cancer, several uncertainties still remain unaddressed. The next most popular disease area (in terms of the number of pipeline candidates) is infectious diseases, of which CDI emerged as the most targeted indication. We observed significant activity (in terms of the number of pipeline candidates being developed) across metabolic disorders and dermatological diseases as well.
Rise in Interest of Non-Industry Players in the R&D of Fecal Microbiota Therapies
FMTs were first used in the 17th century, to treat ruminal disorders in veterinary medicine. In 2015, over 500 cases of FMT procedures were reported to have been carried out in humans, worldwide; of these, 90% demonstrated rapid response and cure rate. Currently, FMTs are being provided by stool banks. These stool banks process the donor material and supply the transplant material to hospitals and institutes where this treatment occurs. Prominent stool banks that are offering FMTs include (in alphabetical order), Enterobiotix, Flora Medicine and OpenBiome.
There are over 15 FMTs under development / commercialized by industry players. Examples of popular developers include (in alphabetical order) Asia Microbiota Bank, Enterobiotix, Finch Therapeutics, MaaT Pharma and OpenBiome. Presently, such therapies are approved for the treatment of recurrent CDIs, specifically in patients who are unresponsive to standard-of-care therapies.
Microbiome Related Initiatives of Big Pharmaceutical Players
Over the years, various big pharmaceutical companies have marked their presence in the field of microbiome-based medicinal products and solutions. The entry of such established companies in this domain was observed to have been via partnering with other microbiome therapy developers, or by making strategic investments in microbiome-focused development initiatives.

Several big pharmaceutical players have partnered with smaller, dedicated microbiome-based therapy / diagnostic developers in order to expand their respective capabilities in this upcoming field of pharmacology. During our research, we observed that Bristol-Myers Squibb (BMS) and Merck have formed numerous partnerships for drugs that are in phase II of development. For instance, BMS signed a deal with Vedanta Biosciences for the development of their lead drug VE303, which is indicated for the treatment of rCDI. Similarly, Merck has partnered with Evelo Biosciences for the latter’s EDP 1503, indicated for the treatment of malignant melanoma.
To get detailed insights about this market, check out the report here
For detailed insights about this domain, check out our report.
Human Microbiome Market: Focus on Therapeutics (including gut-brain axis targeting drugs)
You may also be interested in the following titles:
- China Biopharmaceutical Contract Manufacturing Market, 2020 – 2030
- Continuous Manufacturing Market (Small Molecules and Biologics), 2020 – 2030
- Prefilled Syringe Fill / Finish Services Market, 2020-2030
The post Significant Potential of Gut Flora: Human Microbiome appeared first on Blog.
