For months now, people have been watching closely to see if it’s possible to get re-infected with the coronavirus. It’s taken a while for the signal-to-noise to get better, but by now there’s no doubt that the answer is yes, it’s possible. We’ve just had the first of these in the US, a man in Nevada who was infected twice six weeks apart, with the second round being worse than the first. And in the Netherlands, the first fatality from a reinfection has been reported. All this sounds immediately like bad news, but I’m going to break out the same advice I was handing out yesterday: don’t panic.
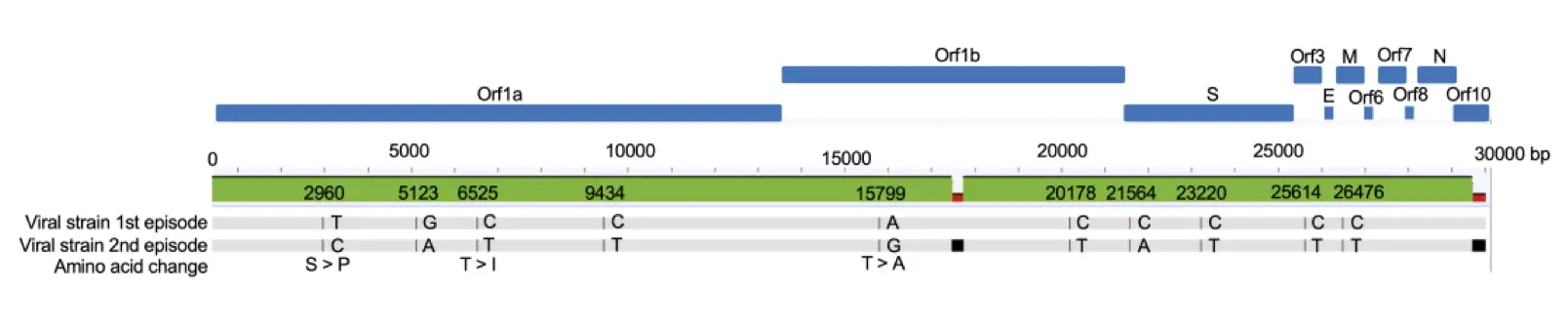
Why not? Because from everything we can see, re-infection is a very rare event. The confirmed examples worldwide could possibly be counted on your fingers (depending on whose count you believe) out of at least 38 million total cases. Looking at the Netherlands case, this was an 89-year-old patient with Waldenström’s macroglobulinemia, a type of leukemia that affects two different varieties of B-cells. She was being treated with chemotherapy to impair. B-cell production, and was thus immune compromised, and the second infection occurred two days after her latest round of treatment. Below is an analysis of the sequences of the first virus and the second – if you’d like more information about what a figure like this means and how to read it, see posts here and here.

You’ll note that there are not very many changes, and that all but three of them are nucleotide changes that make no difference to the actual coronavirus proteins. Let’s take a similar look at the two round of virus in the case in Nevada:

In this case, the red marks at the bottom are noting the changes versus the reference coronavirus genome. Comparing the two, you can see that these two strains had at least eight differences between them, but they’re both considered part of the “20C” clade of SARS-CoV-2, which is a largely North American family. There are several key things to take home from these sequences.
First off, in each of these cases, the unfortunate patients involved were infected by different variants of the coronavirus than they had the first time around. That’s pretty much what you have to show to be sure that it’s a real re-infection – otherwise you’d always wonder if the virus had just never really been cleared the first time. The first-round versus the second-round sequences show some real differences – not gigantic ones, but real. Second, these two second-round viruses were different from each other as well, so it’s not like some particular new supervirus is stomping around re-infecting people around the world. Third, in neither of these reports was it the widely publicized D614G strain coming back around the second time. That mutation really doesn’t figure into either of these cases at all, so watch out for anyone who’s mixing those stories together.
Fourth – and here’s where we start digging into some details – note that the mutations in both of these new re-infection cases have nothing to do with the Spike protein. There’s no change in the Spike in the Nevada sequences (they both had D614G), and the changes in the Netherlands sequences are conserved ones that don’t lead to changes in the protein in that region. Antibodies don’t care about genetic sequences; they respond to the eventual proteins that are displayed, and from what I can see, the Spike proteins of all of these strains are identical.
That’s important for several reasons. For one, the vaccines under development are all raising antibodies and T-cells to the Spike region. That was identified early on as the most promising antigen, building on the work during the SARS and MERS outbreaks. Note also this new paper, a thorough look at the various antibody fractions in patients who have recovered from coronavirus infection. The authors find that Spike-targeting neutralizing antibodies persist out to the limits of their study (five to seven months) while antibodies to the nucleocapsid region (N), which are also raised in most people by infection, disappear more quickly.
This leads to a hypothesis: perhaps in these two cases of re-infection, the patients either did not raise a very robust immune response the first time, and/or raised antibodies more to other antigen proteins compared to the Spike protein. That would account for the second variant being able to slip in under the immunological radar: the antibodies these people used to fight it back the first time were directed towards protein regions that had altered enough to make their recognition less effective.
What about the other confirmed re-infection cases? In the Hong Kong case, there were in fact mutations in the Spike protein, including the D614G, which led some people to wonder if such Spike changes were going to be a general phenomenon and lead to more re-infections. But these two new cases show that it doesn’t have to be that way. Note also that in that case, the first-infecting variant had 58 amino acids missing out at one end (a stop codon mutation in the ORF8 region), which is rather different as well. In the Belgian case, there was one amino acid change in ORF1a in the second variant, three in the Spike (including the D614G), and one in the N protein. The other mutations in the Spike, though, did not match up with the ones in the Hong Kong second variant. And in the Ecuador case, there were nine amino acid changes, but the only one in the Spike region was the D614G.
This is the time to note that a good amount of work has been done on the possible changes in infectivity, etc., of different coronavirus Spike mutants. And we’re not seeing a trend for viral evolution in directions that could increase re-infection or evade the antibodies that are raised by the existing vaccine candidates. The Nevada case, for example, showed the second variant actually had four amino acids that were back to the original Wuhan strain, rather that being something further and further out on the mutational limb. I’ve been looking through the re-infection papers and comparing the mutations seen with that link earlier in the paragraph, and so far anyway I’m not seeing a correlation between the second-round infections and the Spike changes that were flagged for changes in infectivity or antibody susceptibility. (And even some of those possible antibody-resistant mutants identified in that paper above turned out to be equally susceptible to the antibodies raised by the Pfizer vaccine when that team profiled them).
So the situation, for now, seems to be that yes, re-infection is possible. But it’s also quite rare. There are surely cases that we’ve missed, but it’s clearly not something that is happening much. We’re dealing with the fact that the human immune response is hugely variable from person to person – that’s one of its key features. Different people are going to raise different levels of different populations of different antibodies to a coronavirus infection, and that’s a big reason why the clinical course of disease is so variable. Even in these documented reinfection cases, we don’t know the details about what their first immune responses were like (there was no reason to profile these people in such detail the first time!)
Moving beyond that, I would suspect that vaccination, which raises neutralizing antibodies to the Spike protein, will provide a population that is even less susceptible to re-infection than we have in the wild-type-recovered population now, given that three of the five cases we have details of did not have significant changes in the Spike region at all. Now, we don’t know how long vaccine protection will last, or how variable it will be in a broad population – we’re out there getting those data now – but from what we’re seeing, I think the prospects are good. No panic necessary for now.
