The concept of modular building has long been employed in other sectors, but it has only recently acquired traction in the pharmaceutical business over the last two to three decades. Modular construction is a process wherein factory-produced, pre-engineered building units, which are constructed offsite under controlled plant conditions, are assembled on site to construct the final structure. Modular facilities were originally introduced to the pharmaceutical industry at a period when mass manufacturing of tablets and capsules for huge patient populations was the standard; statins were one of the first drugs made in such facilities (prescribed to lower cholesterol). When the patents for these blockbuster pharmaceuticals began to expire in about 2008, the usage of enormous single-product production facilities constructed to satisfy peak demand (during patent exclusivity) began to seem outmoded. Pharma companies are under enormous pressure to bring new drugs to market as soon as possible before their patents expire. The importance of smaller production facilities, which are more efficient and adaptable, has increased as the need to reduce time to market has increased, as has the desire to reduce operating costs and boost manufacturing efficiency. Since modular facilities can swiftly transition between numerous pharmaceuticals and enable formulation and packaging in numerous formats, such as solid, liquid, semi-solid, and parenteral dosage forms, these facilities have become increasingly appealing to drug producers.
With this new set of requirements, pharmaceuticals companies have begun to demonstrate an increased interest in modular facilities and design techniques. Increased demand for multi-product, multi-purpose, lower batch size facilities has led equipment manufacturers to rethink their product lineups.
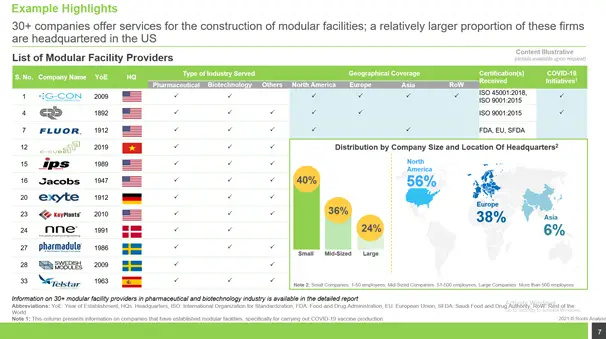
Modular Facility Providers
Presently, more than 30 companies offer modular facility construction services for pharma / biotech industry, majority of which are based in North America.
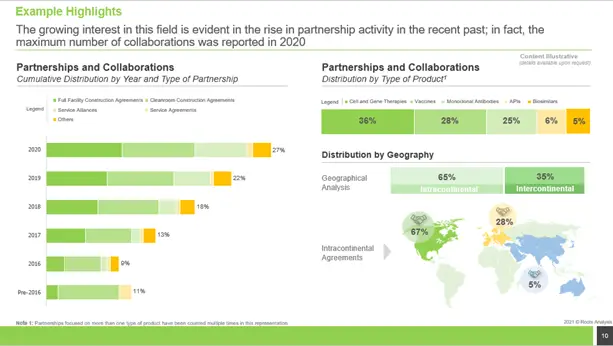
Partnership Trends
The surge in partnership activity in recent years reflects the increased interest in this subject; in fact, the highest number of partnerships was recorded in 2020.
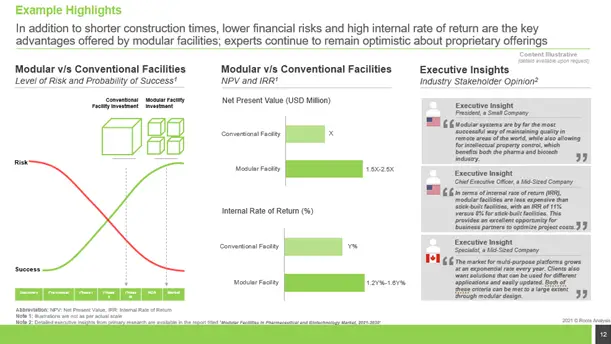
Modular v/s Conventional Facility
Modular facilities provide major advantages such as faster building periods, smaller financial risks, and a high internal rate of return; experts continue to be hopeful about proprietary offers.
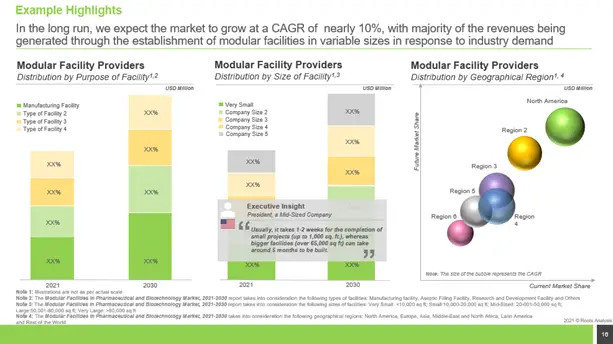
Future Evolution of Modular Facilities Market
Owing to the rise in adoption of modular facilities by pharmaceutical and biotechnology firms, we anticipate the market for modular facility service provider to grow at a CAGR of ~10%.
For further information on this emerging domain, check out the report.
Modular Facilities in Pharmaceutical and Biotechnology Market (2nd Edition)
You may also be interested in the following titles:
- HUMIRA® (Adalimumab) Biosimilars: Focus on Approved & Launched Biosimilars, Investigational & Research Use Biosimilars, Inactive / Terminated / Withdrawn Biosimilars, Industry / Non-Industry Partnerships
- CRISPR Based Therapeutics Market, 2021-2030
- Display Library Technologies and Affiliated Services Market, 2021-2030
The post Modular Facilities: A Paradigm Shift in Pharmaceutical Facility Design appeared first on Blog.
