Fostered by partnerships between national laboratories, academia, and the pharmaceutical industry, the field of nuclear medicines and radiopharmaceuticals has evolved over the past few decades, in parallel with advances in imaging instrumentation, radionuclide production, and radiopharmaceutical development. Nuclear reactors and particle accelerators are presently used to produce a wide array of radionuclides for diagnostic and therapeutic applications. Further, innovative chemistry and automated synthesis devices have been designed to produce a multitude of new radiopharmaceuticals for both imaging and treatment. In addition, high-resolution and high-sensitivity instruments have been developed to assess normal psychological processes, measure the distribution of drugs and monitor treatment effectiveness in living systems.
The recent increase in cancer incidence, coupled to the importance of radioisotopes in the detection and treatment of oncological disorders, are major drivers of the towards radiopharmaceuticals market. As per the World Nuclear Association, over 10,000 hospitals worldwide use radioisotopes in medicine, and about 90% of such procedures are for diagnosis. [RV2] The use of radiopharmaceuticals for diagnosis is increasing at rate of 10% per year[RV3] . Moreover, it has been estimated that within the US, there are over 20 million nuclear medicine-related procedures are conducted per year. This is followed by Europe with 10 million and Australia with 0.56 million of such procedures conducted on an annual basis. Moreover, scientific fraternity worldwide, is continuously working on new radiopharmaceutical products, focusing on optimizing efficacy and minimizing associated risks. [RV4]
Till date, more than 40 radiopharmaceutical products have been approved by the FDA for therapeutic and diagnostic purposes. Specifically, in terms of therapeutic application, which is an emerging segment of this domain, we have observed FDA approval of three novel radiopharmaceutical therapies in last six years. These include, Radium-223 for bone metastases of castrate-resistant prostate cancer, [177Lu]Lu-DOTATATE for neuroendocrine carcinomas, and [131I]I-MIBG for malignant pheochromocytoma and paraganglioma.RV5]
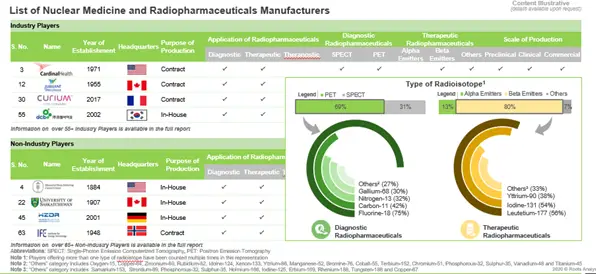
Close to 130 industry and non-industry players, across the globe, claim to have the necessary expertise and supporting infrastructure to manufacture a wide variety of radioisotopes
The production of radiopharmaceuticals involves the handling of large quantities of radioactive substances and chemical processing. While still on a relatively small scale in comparison to the production of conventional pharmaceuticals, it involves a number of aspects that can be quite demanding for small-scale manufacturers. These include the operation and maintenance of processing facilities, complying with the codes of current good manufacturing practices, ensuring effective quality assurance and quality control systems, radioactive material transport, and registration of the products with the relevant health authorities.
Despite the aforementioned challenges, more than 100 radiopharmaceuticals have been / are being developed, using radioisotopes that were either produced by nuclear research reactors or cyclotrons. In order to support the development and manufacturing of these radioactive products, several pharmaceutical companies and academic institutions are developing preclinical, clinical and commercial grade facilities. During our study, we identified over 55 industry players and over 65 non-industry players that are actively involved in the production of nuclear medicines and radiopharmaceuticals.
The growing interest in this field is reflected in the yearly growth in partnership activity
Over the years, several partnerships have been inked between drug developers, radiopharmaceutical manufacturers and other players involved in this industry. We identified around 100 collaboration agreements that have been inked since 2017.
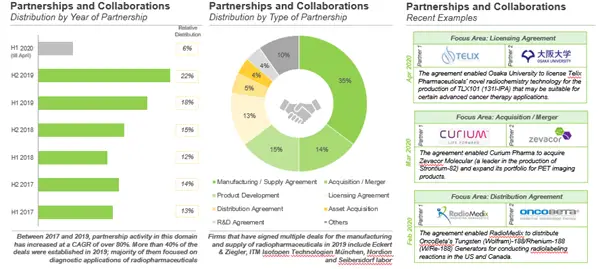
In most of the deals (~35%), the participating organizations have collaborated specifically for the manufacturing and / or supply of radiopharmaceuticals. This is followed by acquisitions / mergers (~ 15%), wherein one company acquired all the assets of another company or merged with another company in this domain. Furthermore, agreements for product development and / or commercialization (15%) and licensing agreement (8%) have emerged as other popular types of deals established in this field, followed by distribution agreement (5%), production asset / facility acquisitions (4%) and R&D partnerships (4%).
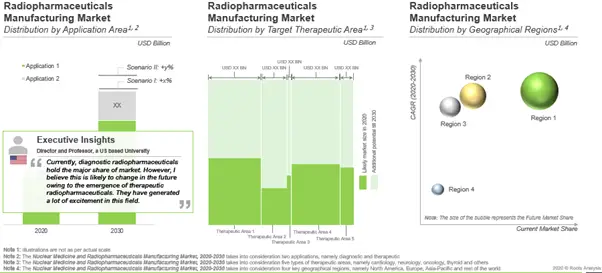
Likely growth of nuclear medicines and radiopharmaceuticals manufacturing market
According to our estimates, the radiopharmaceutical manufacturing market is expected to witness a CAGR of 11%. Sales of radiopharmaceuticals developed for the diagnosis / treatment of cardiovascular disorders is currently the highest contributor to the overall market. In the long term, contributions from radiopharmaceuticals intended for other therapeutic areas, such as oncological disorders, neurological disorders and thyroid are anticipated to increase. North America (primarily the US) is the current leader with the largest share in the radiopharmaceutical manufacturing market.
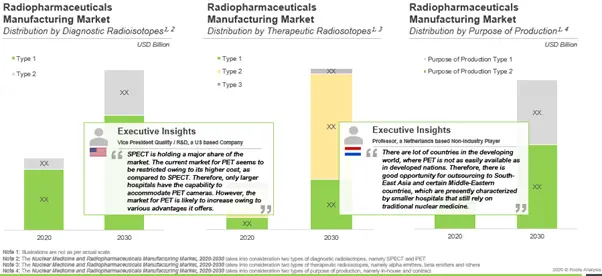
Regarding application area, we believe that radiopharmaceuticals will be majorly used for diagnostic purposes as compared to therapeutic. Amongst the two types radiopharmaceuticals, SPECT radiopharmaceuticals are likely to capture major share of the market in 2030. Within therapeutic radiopharmaceuticals, beta emitters capture the highest share as compared to alpha emitters.
For further information on this domain, check our report here.
RootsAnalysis – Research Insights
You may also be interested in the following titles:
- Microbial Contract Biomanufacturing Market, 2020-2030
- Continuous Manufacturing Market (Small Molecules and Biologics), 2020 – 2030
- Prefilled Syringe Fill / Finish Services Market, 2020-2030
The post Nuclear Medicines And Radiopharmaceuticals: Redefining The Use Of Radioactivity In Modern Day Healthcare appeared first on Blog.
