What You Should Know:
- PhaseV a pioneer in causal machine learning (ML) technology that optimizes clinical trial design and analysis, announced today that it has raised $15 million in funding, led by Viola Ventures and Exor Ventures, including participation from LionBird and a group of prominent angel investors.
- A recent Deloitte study estimates the average cost of developing a single new drug at $2.3 billion in 2022, with an average 7.1 year deployment time. Moreover, the vast majority of drug candidates do not reach the finish line, and many fail the clinical phase even though the biology works.
Optimising Clinical Trial Design to Improve Overall Efficacy
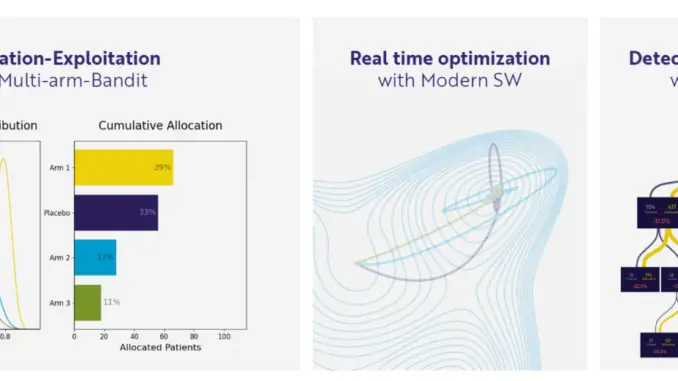
PhaseV tackles this challenge by leveraging proprietary ML technology that provides clinical development teams an advanced ability to retrospectively analyze and optimally design studies, as well as adapt in real-time throughout the trial. This ML-driven adaptive process can significantly accelerate the clinical drug development process and increase certainty along the way, resulting in more efficient, targeted, and ultimately more successful clinical trials.
“Clinical trials are the most time-consuming and costly stages of drug development, and many trials fail due to inherent uncertainties and complexities in trial design and execution,” said Noam Ohana, Managing Director at Exor Ventures,“PhaseV has demonstrated its technological prowess and commitment to reshaping the landscape of clinical trial design and analysis to ensure that promising drugs reach their full potential.”
PhaseV offers two distinct service lines for AI clinical trial optimization. The first includes assessing the potential impact of adaptive trial design on the proposed study, followed by optimal design and execution. The second involves retrospective analysis that detects hidden signals in clinical trial data and evaluates endpoints and subpopulations to redefine success or failure of a trial. Leveraging a wide range of parameters, this multifaceted approach is also valuable for drug repurposing efforts. The company’s approach has proven valuable in a variety of therapeutic areas including oncology, endocrinology, autoimmune diseases, rare diseases and more.