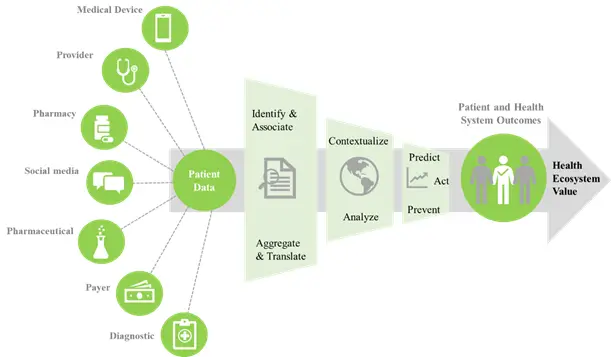
Real world evidence has the potential to support aspects of drug development and commercialization. It can expedite the generation of hypotheses, enable identification of sub-populations with higher risk-benefit ratios to target development efforts, support more efficient and targeted patient recruitment, reduce the burden of data collection and reporting, and lead to earlier conclusions about effectiveness and faster decisions about value and reimbursement. Real world evidence makes possible a new paradigm for closely monitoring the safety and efficacy of drugs prior to and after their approval. This close-monitoring during clinical trials, post-approval commitment phase, or after approval of a subset of patients (through a combination of personal monitoring devices, smartphone apps, phone calls and virtual visits) are likely to benefit patients, researchers and regulators. It is anticipated that the US FDA, European Medicines Agency (EMA) and other regulatory authorities are likely to increase the utilization of real world evidence in influencing the regulatory decisions associated with the safety and efficacy of medical products.
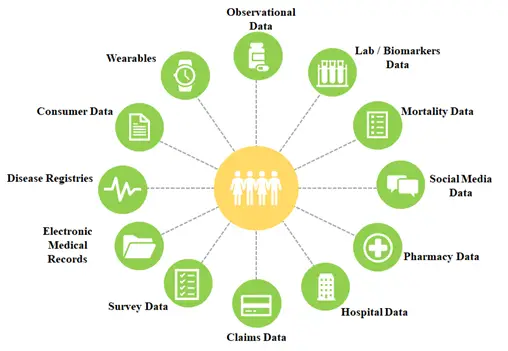
Real World Data Sources
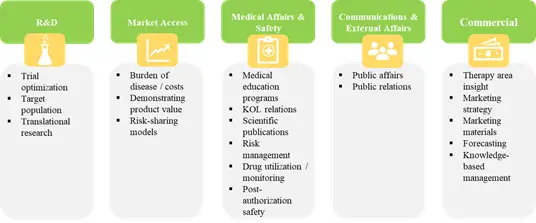
Potential Applications of Real World Evidence
Real world evidence has enormous potential to contribute towards healthcare decision-making, in terms of pre-approval and post-approval guidelines. It can significantly decrease the time and cost of drug development, and can aid in the early stage research in order to discover new innovative treatments. Further, it eliminates the necessity of recruiting clinical subjects to analyze the safety factors of a treatment. If the data generated through real world evidence is of sufficiently high quality, then it can certainly influence the clinical decision-making processes of the regulatory authorities.
Growth Drivers and Challenges
Over the years, industry stakeholders are increasingly utilizing real world evidence in order to reduce costs and optimize operations of their product lifecycle. The numerous potential applications of real world evidence in establishing patient safety standards, ensuring effectiveness of a product and treatment paths, productive market assessment and the disease patterns have further augmented its necessity. Although real world studies have numerous opportunities in the healthcare sector, there are several associated challenges, which act as road blocks to their future growth. The major road block for optimum utilization of real world data is the availability of data in complex or scattered form that has to be structured in order to derive meaningful insights from it.
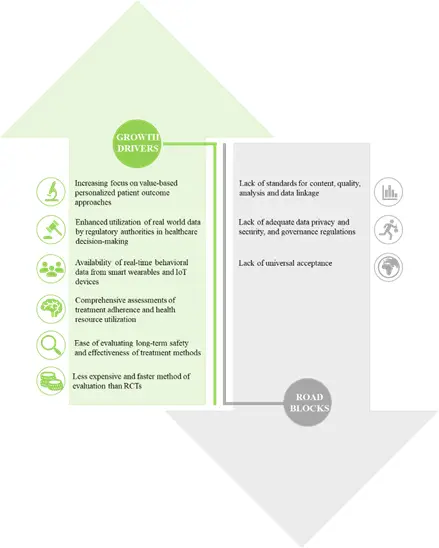
Due to regulatory opportunities and payer demands, the interest in real world evidence in the pharmaceutical industry is continuously increasing. The ongoing advances in compute power and data analytics are allowing stakeholders to gain accurate and reliable insights from EHR data in unique ways. These successful efforts are leading to specific advantages for a given therapy in patient sub-groups.

Check out our new Reports Here-
You may also be interested in the following titles:
- STING Pathway Targeting Therapeutics and Technologies
- Point-of-Care Diagnostics Market for Infectious Diseases by Indication
- Single-cell Sequencing Services and Technologies Market, 2020-2030
The post REAL-WORLD EVIDENCE: THE GAME CHANGER IN DRUG DEVELOPMENT appeared first on Blog.
