What You Should Know:
– THREAD receives an additional $50 million capital commitment from Water Street and JLL Partners to expand its decentralized clinical trial research platform
– THREAD is an innovative technology and service
provider that increases participant engagement by enabling pharmaceutical
companies and contract research organizations to remotely capture data from
participants and sites during, in between and in lieu of in-clinic
visits.
– This major investment comes at a time of incredible
growth for the company, which has expanded its footprint to 40 countries in the
past year. Further, its cutting edge, virtual approach has allowed
pharmaceutical companies and researchers alike to continue with critical
clinical trials throughout the pandemic.
THREAD, an innovative technology and service provider that enables
decentralized clinical research, announced today that it’s accelerating the
company’s expansion after receiving an additional capital commitment of up to
$50 million from strategic healthcare investors, Water Street Healthcare
Partners and JLL Partners. In 2019, Water Street and JLL Partners
invested in and acquired THREAD to further develop and expand THREAD’s offering on a global
scale. The company’s latest capital infusion builds on a year of
significant growth and investments in its platform and services to advance
decentralized research approaches for large-scale, Phase Ib – IV global
clinical trials.
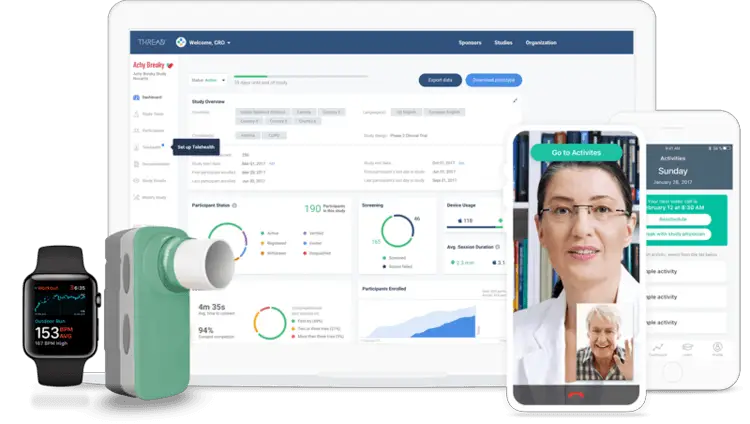
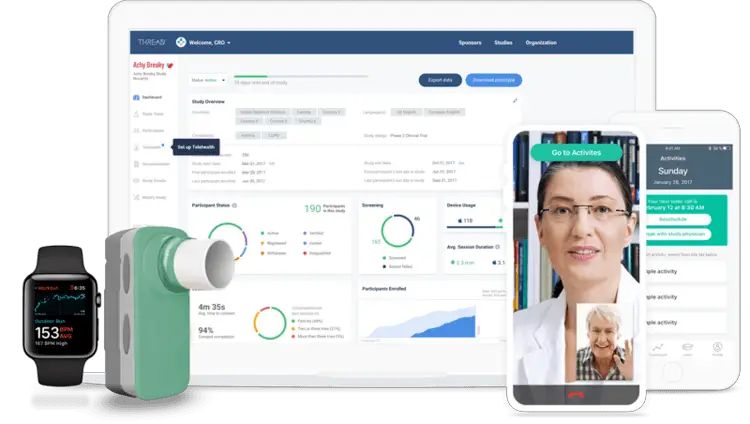
Decentralized Clinical Trial Research Platform
THREAD is a leading provider of a proprietary, decentralized research platform and suite of supporting services used by biopharma, CROs, and life science organizations to remotely capture data from participants and sites during, in-between, and in lieu of in-clinic visits. THREAD’s platform and supporting services are helping customers to reduce study launch timelines, reduce study budgets with Virtual Visits, and bring studies from the clinic to patients’ homes. THREAD provides key platform features such as eConsent, eCOA/ePRO, sensors, reminders, and telehealth Virtual Visits to support remote data capture, hybrid virtual studies, and fully decentralized studies in key therapeutic areas.
Recent Traction/Milestones
Over the past year, THREAD has leveraged the healthcare firms’ combined pharmaceutical expertise, a network of industry resources and capital to:
– expand its global footprint to more than 40 countries;
– add new features to its proprietary, configurable decentralized research platform;
– enhance its capabilities and provide 24×7 global, multilingual customer support;
– rapidly develop and implement COVID-19 clinical trial risk mitigation tools to advance critical research during the pandemic;
– ultimately support customers with virtual visits, hybrid decentralized studies
– fully decentralized studies customized to their individual study needs in key therapeutic areas.
To
date, THREAD has supported more than 100 decentralized studies. The
company will continue to invest in expanding and enhancing its innovative
platform and supporting services as a growing list of the world’s leading
pharmaceutical and life science organizations engage the company as their
partner for their clinical research studies.
