cbaker_admin
Thu, 07/23/2020 – 15:30
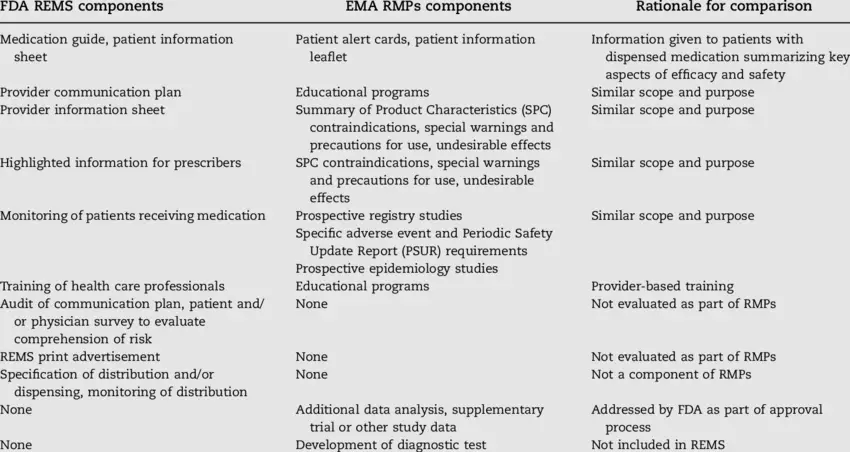
FDA has been empowered since 2007 to require a Risk Evaluation and Mitigation Strategy (REMS) to “ensure the benefits of the medication outweigh its risks.” A comprehensive analysis of the program from inception through 2019 reveals how it has evolved over time. The researchers, from the University of Illinois at Chicago College of Pharmacy and from the Center for Drug Safety and Effectiveness at Johns Hopkins Bloomberg School of Public Health, counted 222 medications that have ever had a REMS designation. Strategies for compliance include requiring pharmacies to distribute medication guides; mandating that manufacturers design communication plans around specific safety issues; and compelling manufacturers to provide “elements to assure safe use” (ETASUs), such as patient registries or prescriber training. Participation has leaned away from the former two, which are less restrictive in nature, with the more stringent latter approach now anchoring the program. That being said, there is little evidence to show that REMS works. Medication guides and communication plans—shown to be ineffective alone at educating patients and providers about drug risks—have been largely weeded out of the program; and problems with the design, conduct, and evaluation of ETASUs linger. However, the study authors report, some assessments indicate that a combination of all three strategies—medication guides, communication plans, and ETASUs—might be a more successful approach.
