But we’re not ignoring it today! There’s a lot of news, a lot of worry, and a lot of speculation about the variant forms of the coronavirus and what that means for the vaccination programs that are underway around the world. The short answer, fortunately, is that the antibody protection you get from the current vaccines still looks solid.
How can I say that, with so much evidence that the antibodies have decreased affinity towards the variant strain proteins? Here’s a new preprint that makes this clear. It shows neutralizing antibody activity in pseudovirus assays against the British (B.1.1.7) and South African (B.1.351) variants, with data versus a panel of monoclonal antibodies, versus convalescent plasma from coronavirus patients, and versus plasma from people who have received the vaccines.
First, the monoclonals. Checking B.1.1.7 against 12 different monoclonals showed that ten of these were equally effective, and two others showed only a small decrease in potency. The B.1.351 variant, though, was tougher. Five of the monoclonals had their activity very significantly impaired, and that includes the Lilly and Regeneron ones that are being used in the clinic right now. B.1.351 seems to evade the Lilly monoclonal outright, although the two-antibody Regeneron cocktail still seems effective as that mixture. This means that anyone using monoclonal antibody therapy with the currently available agents (and those in development as well, also tested in this work) is going to have to keep a close eye out for B.1.351 infections and related strains.
Mapping these activity changes versus single mutations showed that two residues that are trouble are the E484K and K417N mutations. That topic is taken up in more detail in this new paper. It looks at a complete mutational map of the Spike protein’s receptor-binding domain (RBD) and compares the activities of the Lilly and Regeneron antibodies. They picked up on both of the mutations above in the reported variant strains, as well as flagging Y453F, which has shown up in some of the mink-driven variants in Europe. They have also identified some mutations that escape one or the other monoclonal that have not shown up in the wild yet. Mapping all these onto the RBD structure is instructive – there are certainly some patterns that can be rationalized, but as the authors note, there are still mutations in the key RBD binding areas that don’t affect either antibody, and there are also mutations with very noticeable effects that aren’t in direct contact with either antibody at all.
But so far we’re just talking about monoclonals. If you are infected by such a coronavirus, or if you receive any of the current vaccines, you will absolutely not be generating a monoclonal response yourself. No, the whole point of our immune systems is that they come at the problem from a whole set of different directions at once. And in the antibody part of that response, you will make a very long list of different ones, which will in turn be gradually refined and adapted over time. So let’s look at that preprint discussed above and see what happens against convalescent plasma and plasma from vaccinated patients.
Checking plasma from 20 patients who recovered from the coronavirus earlier this year, the authors found that four of them had no loss of potency against either B.1.17 or B.1.351. 16 of the plasma samples showed a drop in potency against B.1.351, and 11 samples showed a drop against B.1.1.7. Those activity drops were 2.7 to 3.8 fold in the latter case, and 11 to 33-fold against the former (more on these numbers in just a moment). Most of that B.1.351 drop seems to be attributable to the E484K mutation. Here’s another preprint that’s just come out looking at convalescent plasma response to the B.1.351 variant and another closely related one, this time using live virus instead of pseudovirus constructs. They also find that the IC50 values are worse in the six patients they examined (more on this below!)
Now to the vaccinated-patient plasma samples, because that’s what a lot of people are really wondering about: how well does being vaccinated with the current agents provide you with protection against the new variants? The authors studied serum from 12 patients that had been given both doses of the Moderna vaccine and 10 patients who had had both doses of the Pfizer/BioNTech one. The activity drop against the B.1.1.7 variant was only about 2-fold in both groups, whereas the overall activity drop against the B.1.351 variant was 6.5-fold in Pfizer vaccinnees and 8.6-fold in Moderna ones.
OK, real-world time. First, those numbers tell us that being vaccinated provides a person with more protection than being infected with the coronavirus itself. That was already thought to be the case for the more common coronavirus forms out there, but it’s good to see that it carries over to these two new variants as well. You will get substantially better protection from being vaccinated, and you don’t have to take your chances with the unpredictable and potentially deadly course of an actual coronavirus infection, either. Now that we have vaccines, the idea of letting the virus just run through a population to achieve immunity by that route looks more obscene than ever. And make no mistake, it has always been an obscene idea as far as I’m concerned. These data also make a case that people who have already been infected naturally and recovered could benefit from being vaccinated, although from a public health standpoint they could be much further back in the line than people who have not yet been exposed.
What about those activity drops, especially the larger ones against the B.1.351 variant? Does that still leave room for protection? Here’s the good news: it very much does. Here’s a graph from the paper I’ve been discussing for most of this post, showing activity against the two new variants versus the good ol’ D614G variant that everyone was worked up about a few months ago:
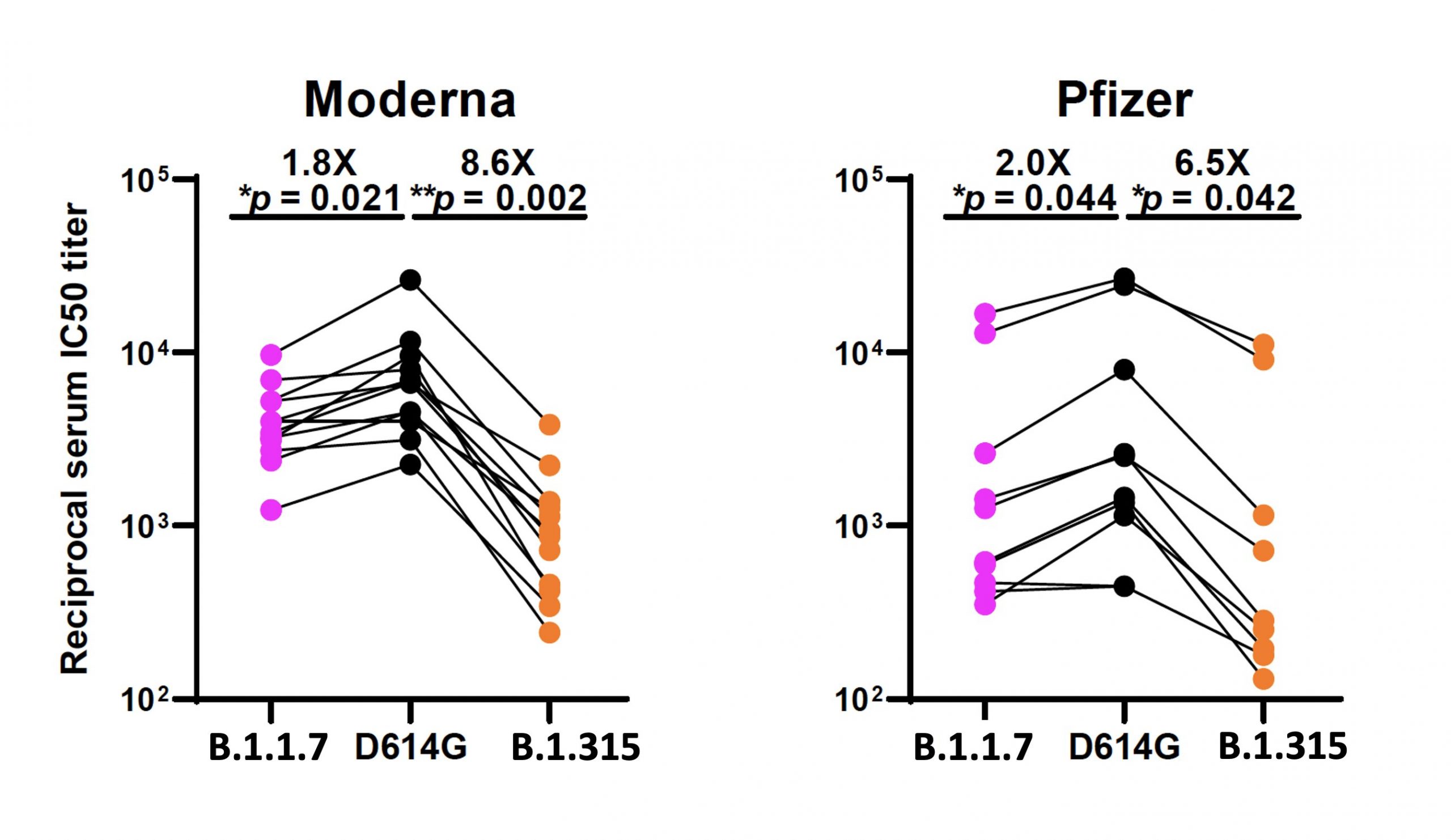
As pointed out by virologist Roberto Burioni this morning on Twitter, it’s important to pay attention to the Y axis on these – it’s a log scale. The bottom of the graph is not a flat-zero no activity line; the bottom is still a hundredfold dilution of the serum from the patients. You have to do that in order to get an assay window to see the differences – straight serum from vaccinated patients should still hammer these viral strains right down. As Burioni says, “There is a decrease, but from extremely high levels. I think these data are very good“. And I agree. It would be very interesting indeed to see this experiment run with plasma from people who have only had the first shot of each of these vaccines, though, wouldn’t it?
The thing we have to watch for, then, are much larger decreases than the sixfold, 11-fold, 30-fold levels. The preprint from the South African team mentioned above is worth considering in that light. It’s a small sample (six patients) of plasma from people who recovered from “first wave” coronavirus, looking at how such antibodies deal with B.1.351 types. Of the six, the activities are 5.7-fold lower, 9.6-fold, 38.1-fold, 53.2-fold, 204-fold, and one that was a complete knockout. Those last two are of concern, for sure, especially the KO. Now, you’d want to see a larger patient sample, to know how common those big drops are. And you’d especially want to see this experiment run versus vaccinated patient plasma, as shown above, which should be better across the board.
What the data are telling us right now is that it definitely looks like vaccination can still handle the variant forms of the coronavirus that we are seeing – but that we also have to be on our guard, because there is no law that says that this protection can’t be breached. Taking public health measures to decrease the spread of the new variants is critical, as is getting as many people vaccinated as quickly as possible. If we mess either of those up, we are asking for serious trouble.
But there’s even a potential way out of that trouble, although you’d hate to have that emergency and to break that particular glass. As Moderna has said, variant mRNAs can be turned out quickly to be formulated as a new vaccine. Putting one together with the E484K and K417N point mutations (and others) should be basically the same process as what’s been used to make the currently administered ones (both Moderna and Pfizer/BioNTech). You would take a hit in production, of course, because you’d have to stop the current forms and start up the new ones. And you would be taking a small-but-real risk that these might perform differently in adverse events (but this would still be similar to the way that we roll out different flu vaccines every year). So the mRNA technologies offer us a potential counterattack, which is good – but let’s try not to have to use it!
