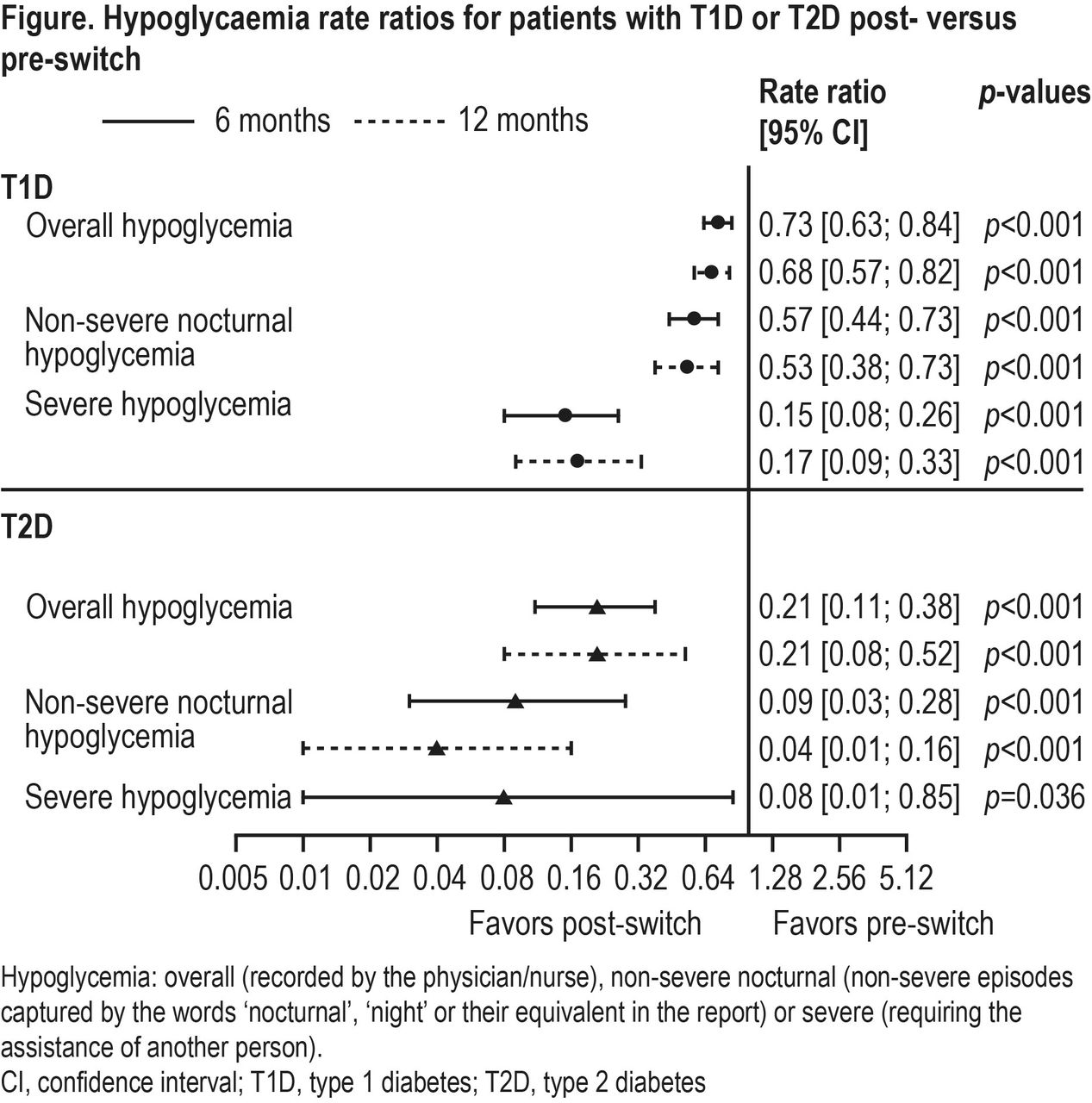
Annals of Pharmacotherapy, Volume 54, Issue 9, Page 846-851, September 2020.
Background: Basaglar, insulin glargine (BGlar; Eli Lilly, Indianapolis, IN), a follow-on biologic, was developed after the patent for Lantus, insulin glargine (LGlar; Sanofi-Aventis, Paris, France) expired. Objective: To compare the dosing and hemoglobin A1C (A1C)-lowering effects of BGlar compared with LGlar in a real-world setting. Methods: Adult patients, at 5 clinics, with type 1 (T1DM) or type 2 diabetes mellitus (T2DM) who were converted from LGlar to BGlar were included in this retrospective observational study. The primary outcome compared mean basal insulin dose (U/d) from the date of conversion to 6 months. Basal insulin and total daily insulin doses were also compared from baseline to 3- and 12-months postconversion, as also change in A1C, body weight, and estimated monthly acquisition costs of basal insulin. Results: Of the 225 patients included, 56% were male, and 81% had T2DM. The mean conversion dose (U/d) of LGlar was 46.3 ± 32.7. There was no significant difference in the mean BGlar dose (U/d) at 6 months (45.9 ± 33.5; P = 0.52), nor was there a statistical difference at 3 or 12 months. There were no significant differences in change in A1C at any time point. The estimated monthly acquisition cost of BGlar was significantly less than that for LGlar at conversion ($286 vs $341, P < 0.001) and 6 months ($290 vs $351, P < 0.001) respectively. Conclusion/Relevance: The results of this retrospective study suggest that BGlar resulted in similar glycemic outcomes compared with LGlar in a real-world setting and may be a preferable option in a value-based health care environment.
Background: Basaglar, insulin glargine (BGlar; Eli Lilly, Indianapolis, IN), a follow-on biologic, was developed after the patent for Lantus, insulin glargine (LGlar; Sanofi-Aventis, Paris, France) expired. Objective: To compare the dosing and hemoglobin A1C (A1C)-lowering effects of BGlar compared with LGlar in a real-world setting. Methods: Adult patients, at 5 clinics, with type 1 (T1DM) or type 2 diabetes mellitus (T2DM) who were converted from LGlar to BGlar were included in this retrospective observational study. The primary outcome compared mean basal insulin dose (U/d) from the date of conversion to 6 months. Basal insulin and total daily insulin doses were also compared from baseline to 3- and 12-months postconversion, as also change in A1C, body weight, and estimated monthly acquisition costs of basal insulin. Results: Of the 225 patients included, 56% were male, and 81% had T2DM. The mean conversion dose (U/d) of LGlar was 46.3 ± 32.7. There was no significant difference in the mean BGlar dose (U/d) at 6 months (45.9 ± 33.5; P = 0.52), nor was there a statistical difference at 3 or 12 months. There were no significant differences in change in A1C at any time point. The estimated monthly acquisition cost of BGlar was significantly less than that for LGlar at conversion ($286 vs $341, P < 0.001) and 6 months ($290 vs $351, P < 0.001) respectively. Conclusion/Relevance: The results of this retrospective study suggest that BGlar resulted in similar glycemic outcomes compared with LGlar in a real-world setting and may be a preferable option in a value-based health care environment.
