Shots:
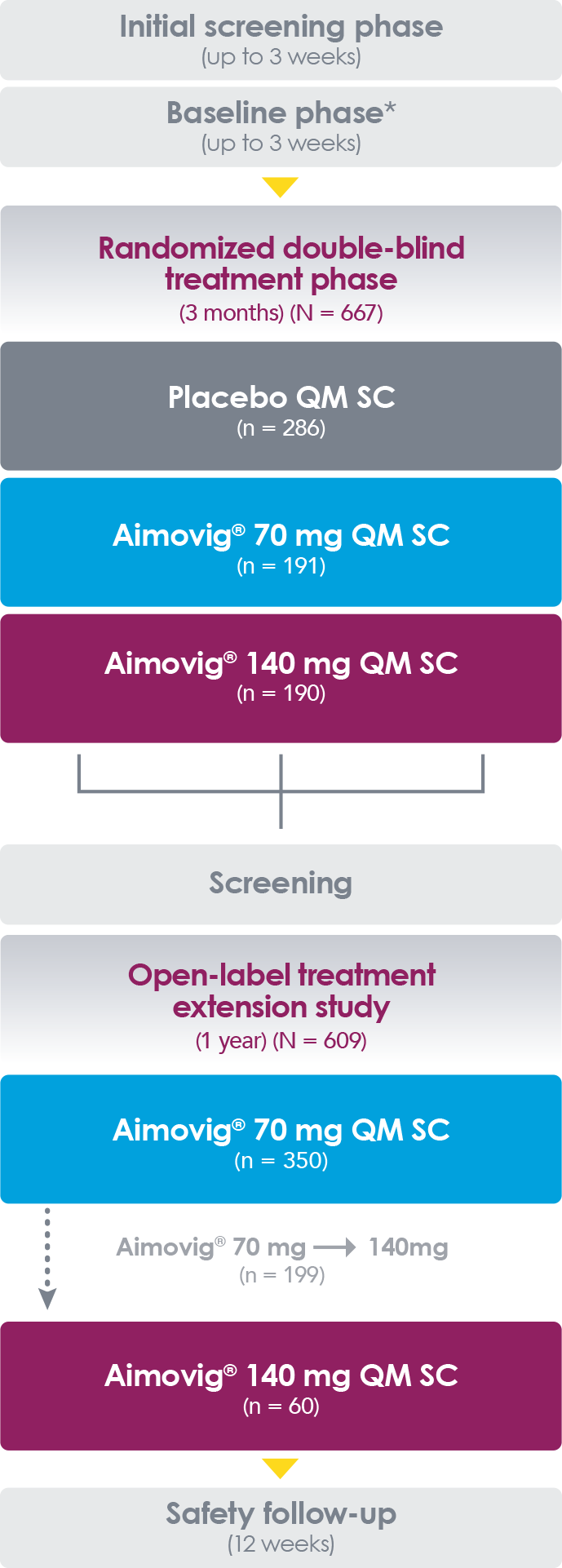
- The OLE P-II study involves assessing of Aimovig monthly (70mg) vs PBO in 383 eligible adult patients with an episodic migraine for 12wks. 250 patients increasing their dosage to 140mg monthly after a protocol amendment to assess the long-term safety of the higher dose
- The results of the OLE study showed the therapy helped patients to achieve 5.3days reductions in MMD and 4.4 days reduction in the use of AMSM, and has the longest duration of safety and efficacy trial data for any anti-CGRP pathway therapy, will be presented at the Migraine Trust Virtual Symposium
- Amgen will also present additional studies that include interim results of the LIBERTY OLE study, as well as efficacy and safety results of Aimovig in the EMPOwER study. Aimovig is the first and only FDA and EMA-approved migraine preventive treatment targeting CGRP receptor
Click here to read full press release/ article | Ref: Amgen | Image: CNBC
Related News: Amgen’s Aimovig (erenumab-aooe) Receives FDA’s Approval for Prevention of Migraine in Adults
The post Amgen Report Five Year Data of Aimovig (erenumab-aooe) in Open-Label Treatment Period of P-II Study for Episodic Migraine first appeared on PharmaShots.
