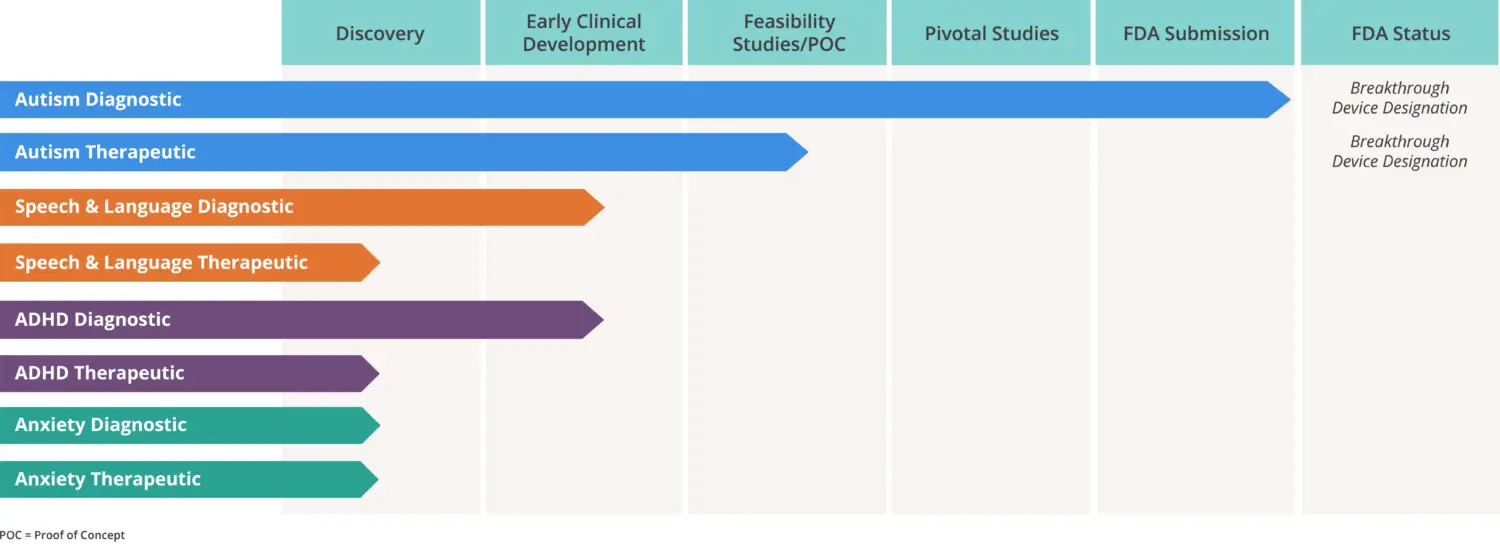
What You Should Know:
– Cognoa, a pediatric behavioral health company developing diagnostic and therapeutic solutions for children living with behavioral health conditions, has been granted FDA authorization to market the company’s first-of-its-kind, AI-based autism diagnosis aid – Canvas Dx.
– Canvas Dx is the first diagnosis aid designed to help physicians to diagnose autism spectrum disorder (ASD) in the primary care setting, as well as the first diagnosis aid for ASD authorized for commercialization by the FDA.
– Harnessing AI and Machine Learning technology, Canvas Dx is able to reference thousands of external data points and simultaneously assess hundreds of features of a child for predictive autism signals. Furthermore, Cognoa’s AI algorithm has been trained on data from a wide range of children from various demographics and has been consciously built to embrace gender, racial, ethnic and socio-economic origins to facilitate a more efficient, equitable pathway to care for children at risk for developmental delay and their families.
Why It Matters
Autism is an increasingly common neurodevelopmental condition that today affects one in every 54 children in the United States, a 178% increase in prevalence since 2000. While autism can be reliably diagnosed in children as early as 18 months, the average age of autism diagnosis has remained at 4 to 5 years old for decades. Non-white children, females, and those from rural areas or disadvantaged socio-economic backgrounds are often diagnosed even later, or missed altogether. This reality means many children miss a critical neurodevelopmental window when early diagnosis and subsequent early interventions have the greatest potential to improve lifelong outcomes. Disparities in autism diagnosis are largely due to the lack of female and diverse representation in autism research to date. In addition, the reliance on diagnostic assessments that are time-consuming to administer within the specialty care setting and the growing shortage of diagnostic specialists contribute to the average three year delay between first concern and diagnosis.
“The current system is already at a breaking point as it is unable to provide early diagnoses and intervention opportunities to many children and families. With rapidly-rising autism rates, this crisis will only worsen without new approaches and innovations,” said Dave Happel, CEO of Cognoa. “The FDA authorization of our diagnosis aid, Canvas Dx, is a significant milestone in Cognoa’s development and a crucial step towards making early diagnoses more accessible to children and families – regardless of gender, ethnicity, race, zip code, or socio-economic background. We look forward to partnering with the healthcare community as we introduce Canvas Dx in the coming months.”