Over the last few decades, the pharmaceutical industry has been using batch processes for production of pharmaceutical products, which is a multi-phase process. However, the surge in the demand for drugs due to COVID-19 has led the pharmaceutical industry to shift on to a more agile and flexible technique- continuous, which is a single flow process allowing the industry to scale up quick, manufacture easily and shortens the drug supply chain. In fact, the USFDA has categorized continuous as one of today’s most important tools for modernizing the pharmaceutical industry.
Advantages of Continuous Manufacturing
Some of the key advantages are highlighted below:
- Reduction in manufacturing cost (by 15-30%)
- Reduction in manpower (by 50-70%)
- Lower product deviation (by 50%)
- Smaller footprint requirement (by 50-70%)
- Reduction in power consumption (by 40%)
- Faster scale up.
Moreover, the continuous processes use advanced sensors, and in-process analytics that enable the measurement of critical parameters and processing conditions in real-time.
Highlights on comparison of batch manufacturing Technique and Continuous Manufacturing Technique
| S. No. | Characteristics | Batch manufacturing technique | Continuous manufacturing technique |
| 1 | Cost of production | Relatively a slower process; increases the cost of production | Comparatively a faster process; reduces the cost of production |
| 2 | Time efficiency | Requires shutting down of the whole equipment on a regular interval; makes the process, time consuming | Does not require shutting down the equipment; time-efficient process |
| 3 | Energy requirements | High energy consumption while starting the equipment | Lesser energy consumption being a continuous process |
| 4 | Manpower required | Requires multiple human operators for the production of each batch; the risk of human errors is significantly high | Requires much less human attention; reduced human errors |
| 5 | Continuous monitoring | Monitoring of batch is only possible after the completion of process | Monitoring is possible due to PAT and closed-loop automated process |
| 6 | Batch / lot generation | Specific number of batches are generated at a time | Batches are generated as per the demand |
| 7 | Floor-space requirement | Larger floor-space required | Lesser floor-space required |
| 8 | Batch-to-batch inconsistencies | High risk of batch-to-batch inconsistencies | Low risk of batch-to-batch inconsistencies |
List of Companies Offering Continuous Manufacturing
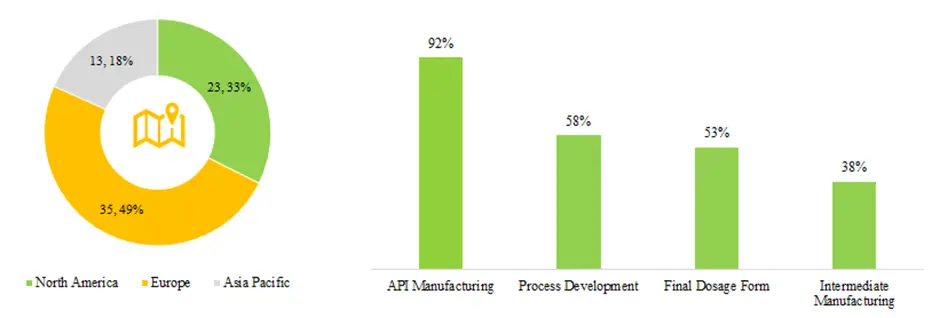
Currently, over 70 companies across the globe claim to offer continuous manufacturing services. Over 90% of the companies are engaged in offering services for API manufacturing, while only 53% of the companies offer the services related to final dosage form. Furthermore, it is interesting to note that 49% of the continuous manufacturing players are predominantly present in Europe.
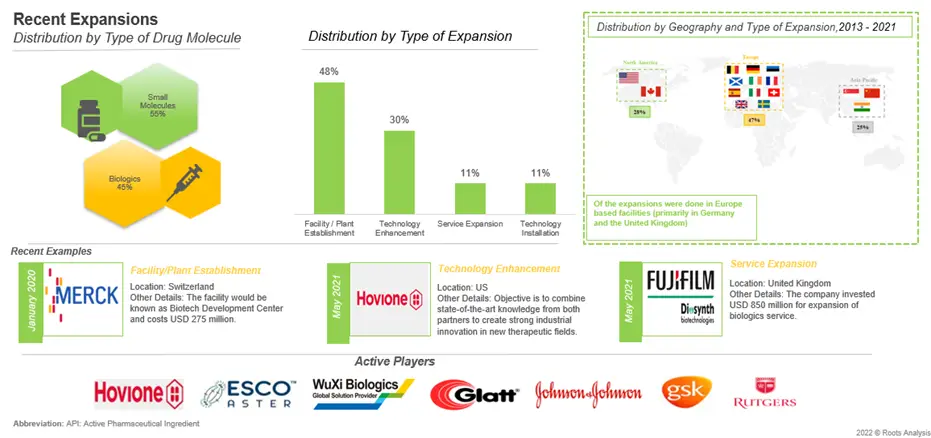
There has been a significant increase in the number of expansions in order to adopt continuous manufacturing since 2016. Majority of expansions were signed in the Europe (primarily in Germany and United Kingdom). Moreover, close to 50% instances were related to plant establishment followed by technology enhancement (30%).
Adoption of Continuous Manufacturing
As per the interviews conducted with industry stakeholders, the industry is currently in the initial stages of adopting continuous manufacturing. However, the ongoing COVID-19 crisis is likely to accelerate the industry’s shift to these systems. In fact, a number of established big pharma players have already adopted these systems.
Bottom-Line
Keeping every trend in mind, Roots Analysis has provided complete information on market trends in continuous manufacturing domain, which has some of the very recent and precise activities listed for the clients to help them make better decisions in its upcoming report titled, Continuous Manufacturing (Small Molecule and Biologics) Market, 2nd Edition, 2022-2035. To find answers to key decision-making question and to know further about the market forecast analysis, highlighting the likely growth of the Continuous Manufacturing (Small Molecule and Biologics) Market, for the time period 2022-2035.
For more details on this emerging domain, check out the following report:
Continuous Manufacturing (Small Molecules and Biologics) Market
You may also be interested in the following titles:
- Single-Use Upstream Bioprocessing Technology
- Pharmaceutical Secondary Packaging Market
- Viral Vaccine Cell Culture Media Market Distribution
Our Social Media Platform
Web: https://www.rootsanalysis.com/
LinkedIn: https://in.linkedin.com/company/roots-analysis
Twitter: https://twitter.com/RootsAnalysis.com
The post Continuous Manufacturing: A Magic Bullet to Meet the Demand for Pharmaceutical Products appeared first on Blog.
