Ensuring Data Security and Compliance in Pharma’s Digital Transformation
Quicker than a storm, a pandemic has left us in a black hole of confusion and uncertainty. On this matter, pharma could be compared to a large ship that suddenly has lost its navigation in a stormy sea of changes that a new wave of digital has brought along. Since then, information security and consent management issues have become much more urgent and complex.
The events like Next Normal Week exist to give us a landmark on how to navigate such turbulent times. We were excited to be a part of this event and share our expertise on how to secure pharma companies in terms of regulation and management of personal data thanks to the best DCF security standards – one of the most hot-button topics of 2021.
Read on to summarize the key findings of our session at Next Normal Week and discover the best practices and technical expertise that provide a robust, hyper-secure ecosystem “trained” to respect information security.
Introduction
Practically all areas of the pharmaceutical industry are entirely dependent on patient and HCP data and consent gathering. Thus, pharmas that are perfecting up their content production workflows with Digital Content Factory-type organizations in place experience growing concerns about data security and access management.
The rapid shift towards digital provides many opportunities for ameliorated content management, but at the same time, it contains a lot of risk of information leakage – which has always been one of the biggest threats for pharmaceutical companies. It comes along with a snow globe of laws and regulations that every year only grows.
When people are talking about gathering consent, they think about compliance. It includes multiple areas: General Data Protection Regulation (GDPR), California Consumer Privacy Act (CCPA), and other regulations. The other important layer is Personal Data Management that includes gathering and storage of users’ consents, usage of systems that help us to get it (Web Forms, CRM systems, and others), Data Flow, Cyber Security, and Data Protection – the essential elements of compliance, consent gathering and other regulations.
User experience (UX) is often overlooked but it is an important point for modern customers who are more than ever concerned with the security of their personal data. This process must be transparent enough as according to statistics, 95% of customers say they are more likely to be loyal to a company they trust.
At the level of UX it is important to provide opportunities for Consent Gathering (Opt-in, Forms, Notifications), Terms of Use and Privacy Policy, Data Flow, Data Termination (Opt-out, Unsubscribe, Notifications), Right to be Forgotten.
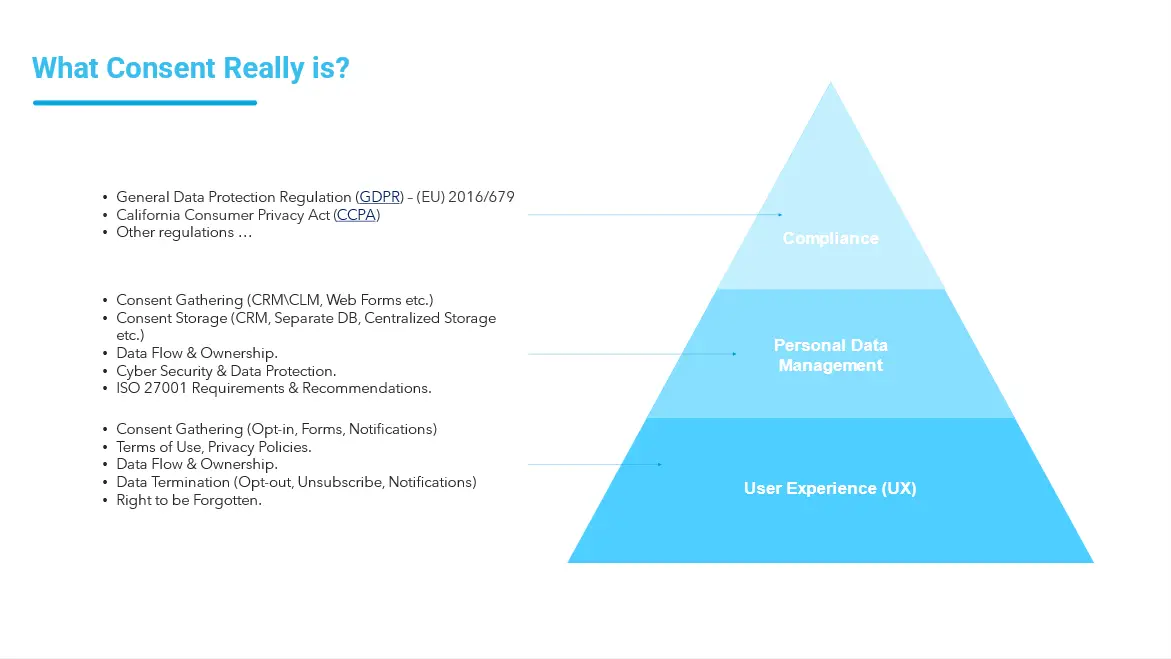
In such a highly regulated environment, pharma should apply all digital talents and tools to manage all processes in terms of data security and compliance management and provide robust trainings to boost regulatory compliance.
How to Establish an Effective Data Compliance System
Security of medical database
According to the IntSights report, about one-third of healthcare databases stored both locally and in the cloud are currently exposing sensitive patient data. The factors like misconfigured databases are among the riskiest when it comes to the security of Patients’ and doctors’ data. The problem is that many healthcare providers have continued to shift data and other assets online without prioritizing investments in cybersecurity tools or procedures to prevent the leakage of sensitive information.
Possible reasons to worry may include the desire to expand the functionality of certain tools without the intervention of certified developers. In some cases, the limitations of the budget or wrong choice of tech providers may push to unacceptable practices, such as reverse engineering or unauthorized extensions, which make data vulnerable. So, it is strongly recommended to involve certified professionals whenever dealing with a licensed system.
In terms of access, when all businesses are especially concerned about the security of their data, things like multi-factor authentication are self-evident. But security-oriented companies may also limit the number of accesses to the database, reduce it to specific IP addresses and allow just manual review or confirmation system to minimize the chance of leakage.
Security of content
The question of security in the pharmaceutical industry is urgent, however, the customers’ expectations also include coherent messaging and best-in-class content. The secure production of pharmaceutical content needs a certain protocol of the required measures and regulation. That’s why industry leaders are currently looking for a solution that can automate these processes.
When pharma has an idea of a new project, they need an agency to turn it into reality. But it also comes with risks, as a lot of the data contained in the marketing and promotional materials are sensitive for the pharma companies.
Meta tagging greatly simplifies the search of the necessary information in DAM, but a much better solution in terms of data security is the establishment of accesses hierarchy, where each user role includes a certain level of access to the certain information.
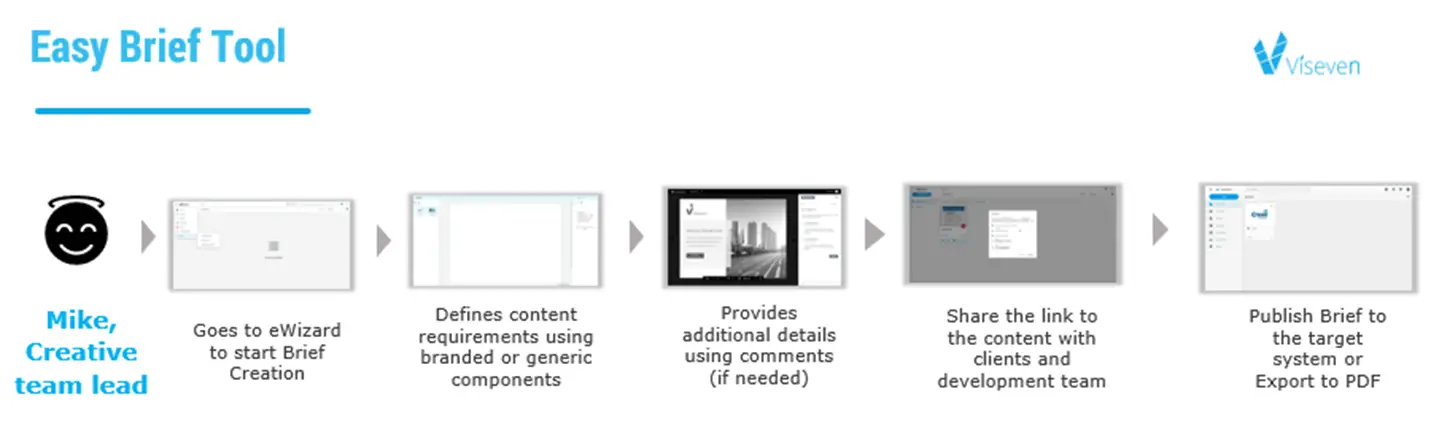
Also, to provide additional security when working with content, agencies can sign an NDA agreement and be monitored with help of a briefing tool that allows getting the best from a pharma-agency partnership. A comprehensive brief is very important, but it’s not always transmitted in a secure manner. It is much more convenient to use tools designed specifically for briefing, and that are in-built into the platform for working with content.
The EasyBrief Tool from eWizard can turn your brilliant ideas into brilliant campaigns. It was designed to improve your business flow by systemizing everyday work with customers. Easy Briefing tool gives a unique opportunity for brand managers to brief agencies just in several clicks. Pre-approved modules can be used to visualize the task, comment, and co-create with the agency.
Consent Management
Now, for the most part, consent gathering process is happening at the meetings with HCP. This happens as follows: med rep asks HCP if they are willing to receive updates from the pharma company. If the HCP is ready to share the personal information – the consent gathering process starts (see scheme 1). After that, all the data is going to the CRM or Marketing Automation system and the HCP start getting bombarded by traffic from the pharma company. This is the model that is basically associated with all channels simultaneously. The problem that it does not allow differentiate the initial interest of the HCP and provide a targeted communication where each message presents a real value for the customer.
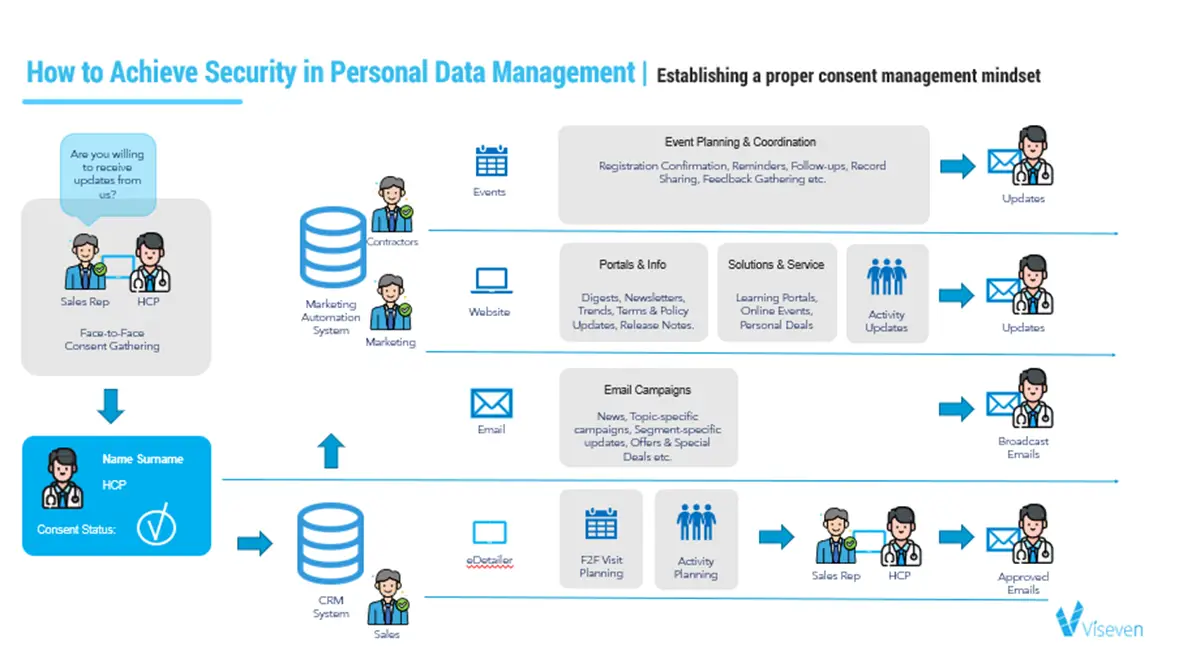
Scheme 1
Where the change of mindset should happen
As usual, everything starts with a face-to-face visit but further, but further, a more efficient model can be suggested. Instead of getting a single tick from the HCP that they are willing to receive further information on one channel, HCP is getting a full detailed list of the options you are offering as the service. From the moment on, the HCPs are getting targeted information that is based on their initial interest. For example, if the HCP ticks a face-to-face visit, the information goes straight to the CRM system or the Consent Management system, depending on the infrastructure that the company is using. Further, a sales rep can process this information and plan the eDetailer presentation and further activities accordingly.
If the HCP chooses to receive a broadcast email, it brings the whole integration with a marketing automation system. Further HCP can get the various types of marketing information from marketers, contractors, and other people who are in charge of this process. This process practically changes the name of the game and gives the customer a broad picture of what they are signing up for and what type of information they can expect from the pharma company in exchange for consent.
However, the other cast-iron rule is that the customer, at any minute, has a right to unsubscribe from the information they previously agreed to receive. That is why the second point changing the mindset in the consent management process is that using this model the customer may choose to stop communication through one channel, let it be broadcast emails (see scheme 2) but will not stop receiving the information from other channels like portals, events and so on. Using this checkbox, the company may deactivate any type of undesirable activities without interrupting an entire communication with the HCP.
This way, we can generate the golden rule of consent management: the customer should always be provided with transparency on what their data is used for, what channels will be engaged, and see what’s behind each tick they put into a checkbox.
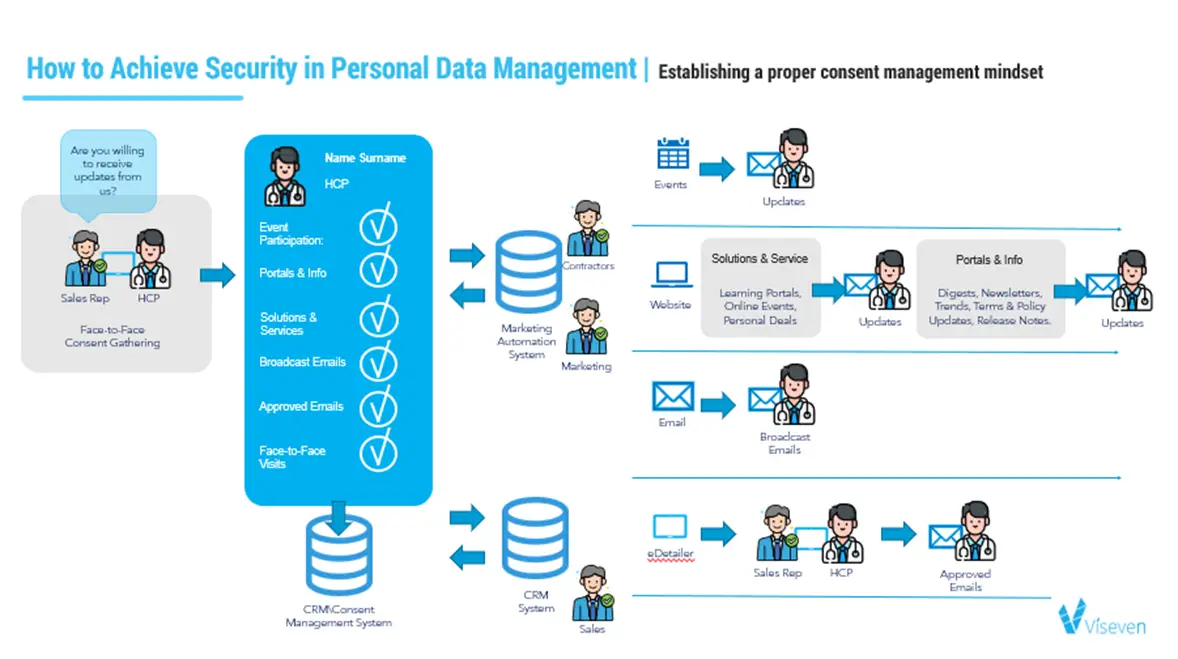
Scheme 2
ISO Certification
Pharmaceutical companies manage a huge amount of sensitive information cannot be disclosed. Today the need to remain competitive and provide a secure environment for the pharma customers prompt us to be certified against a strictly defined and delineated standard that is ISO/IEC 27001
The International Standards Organization (ISO) means that the company implemented a number of procedures, policies, and guidelines that allowed it to attain the full range of control and protection over the Company’s assets.
Viseven has successfully passed the first supervisory audit ISO 27001. Among the main advantages of adhering to the completed certification norms are asset protection from leakage, theft, or loss; higher trust from the Customers, as well as Employees’ confidence. All of this ensures our greater strength in the highly competitive landscape, with clear and transparent workflows, as well as role distribution among our own specialists.

Request the video recording of our session at Next Normal Week to hear about Viseven’s best findings on how to run digital transformation in a secure and compliant environment. In case you have questions concerning our expertise in establishing a robust hyper-secure ecosystem – turn to our experts for insights and guidance.
The post Ensuring Data Security and Compliance in Pharma’s Digital Transformation appeared first on Viseven.

