What You Should Know:
– Scopio Labs announced FDA clearance of its AI-powered
X100 microscope and decision support system with Full Field Peripheral Blood
Smear (Full Field PBS) application.
– Using advanced computational photography imaging and tailored AI tools, Full Field PBS gives clinical laboratories an unprecedented ability to capture digital scans with full-field view of the monolayer and feathered edge at 100X oil immersion resolution level.
– The global market for hematology analyzers and reagents
is currently $7.6 billion and is expected to reach $10.6 billion by 2025.
Scopio Labs, a leading provider of Full Field Morphology (FFM), announced that it was
granted FDA clearance to market and sell its X100 with Full Field Peripheral
Blood Smear (Full Field PBS) Application, unlocking the potential of in
vitro hematology diagnosis. Full Field PBS is also available in Europe with CE mark certification granted earlier this year.
Why It
Matters
Blood is one
of the most foundational gateways to health information. Roughly 120,000
laboratories worldwide conduct 600 million PBS tests annually for the global
population, predominantly via manual microscopes. Even with the adoption of
digital tools, today’s solutions do not showcase all required regions of
interest in a PBS slide, only capturing snapshots of cells. Consequently,
technologists frequently default to the manual microscope for a more detailed
examination of the raw data.
AI-Powered
Full Field Peripheral Blood Smear (Full Field PBS) Application
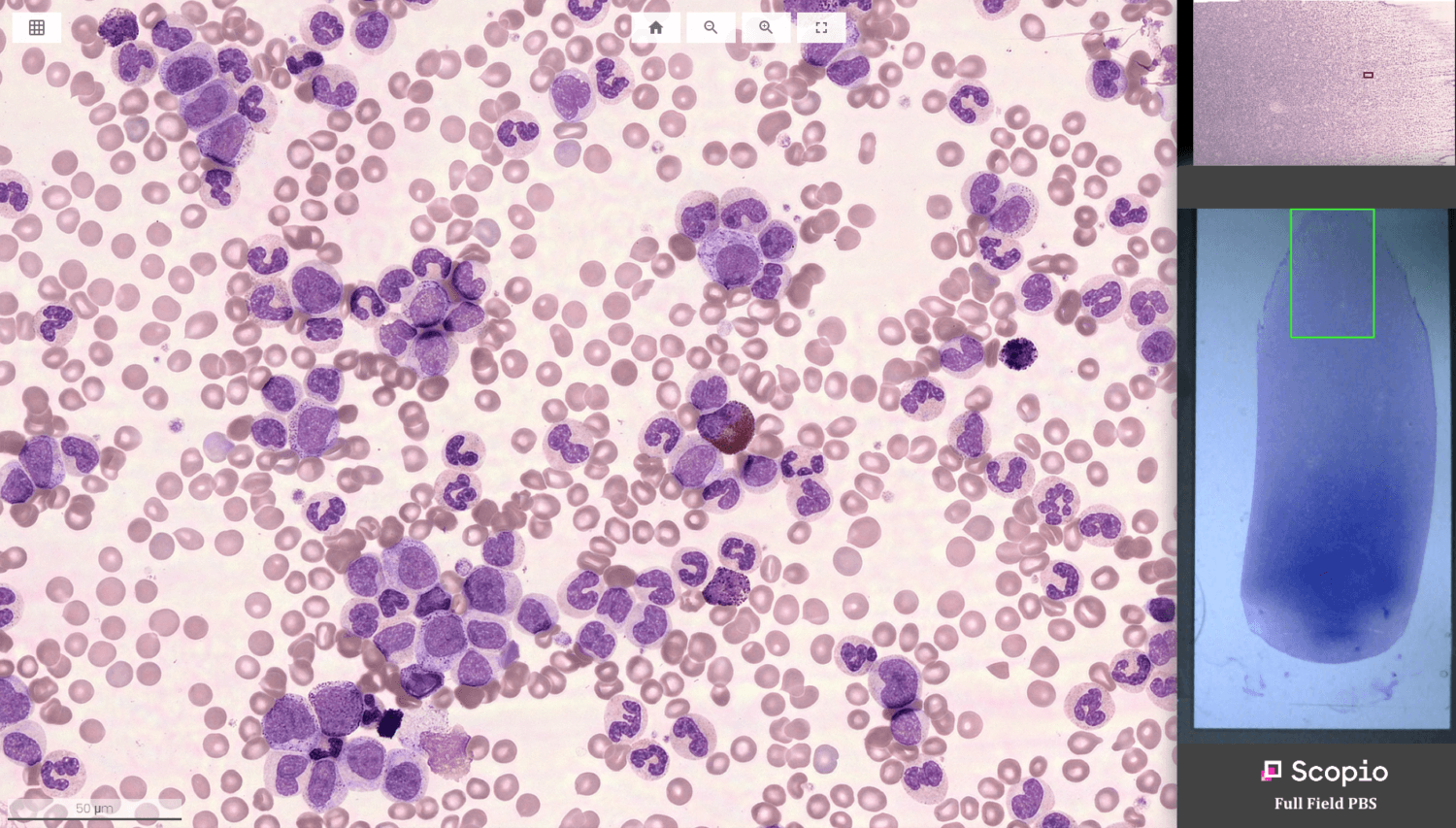
To help improve diagnostic accuracy leveraging novel computer vision tools, Full Field PBS gives clinical laboratories an unprecedented ability to capture digital scans using advanced computational photography imaging and tailored AI tools. The Full Field PBS utilizes adaptive monolayer identification in support of long and short smears and automates the analysis process by pre-classifying 200 white blood cells (WBC), providing platelet pre-estimate, and enabling RBC morphology evaluation. Accelerating routine analysis, Full Field PBS includes a tailored decision support system for pre-classification of white blood cells into 16 classes, red blood cell morphology evaluations, platelet location and count.
“Understanding the challenges lab technicians, hematologists and hematopathologists face when evaluating blood samples containing large numbers of morphologically-unique cells in a timely fashion, we designed our solution specifically for hematology labs where we can improve quality of care, consistency of results and reduce review time,” said Scopio Labs’ CEO and Co-Founder, Itai Hayut. “We are thrilled to receive FDA clearance following the successful completion of a multi-center study, as we bring our innovative solution to laboratories around the U.S. to help improve the outcome of diagnosis and care.”
Recent Traction
Earlier this
year, the company closed a $16 million Series B funding round, bringing total funding to $30 million. Scopio Labs is setting its sights
on transforming all hematology applications, including bone marrow aspirates
(BMA) and body fluids. With a robust product development pipeline, and the
ability to detect morphological events on a cellular and subcellular scale,
Scopio Labs will open the door for morphology-based diagnostics, disease
monitoring and treatment adjustment for various blood cancers.
