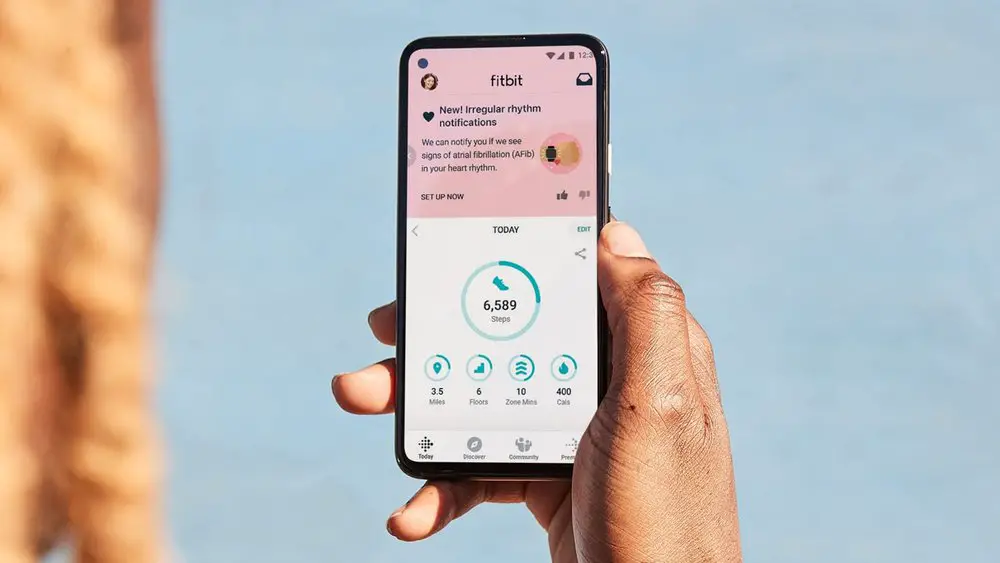
What You Should Know:
– Fitbit announced it has received clearance from the FDA for its PPG (photoplethysmography) algorithm to identify atrial fibrillation (AFib), a condition that affects more than 33.5 million people globally.
– The algorithm will soon be available to users in the U.S. through Fitbit’s new Irregular Heart Rhythm Notifications feature on eligible heart-rate tracking devices. We can share the full list at feature availability.
PPG AFib detection Benefits
AFib is a form of irregular heart rhythm that affects nearly 33.5 million people globally, and individuals with AFib have a five times higher risk of stroke. Unfortunately, AFib can be difficult to detect as there are often no symptoms and episodes can come and go. PPG algorithm. PPG AFib algorithm can passively assess a patient’s heart rhythm in the background while they are still or asleep. If there’s anything that might be suggestive of AFib, you’ll be notified through our Irregular Heart Rhythm Notifications feature — allowing you to talk with your healthcare provider or seek further assessment to help prevent a significant medical event, such as stroke.
Why It Matters
The Irregular Heart Rhythm Notifications feature has the potential to make a significant impact on the detection and treatment of AFib.
The unique capabilities of Fitbit devices — especially its 24/7 heart rate tracking and long battery life — can accelerate identification through long-term heart rhythm assessment. Fitbit’s wide range of devices — available in different form factors, at multiple price points, and with cross-platform compatibility — will make irregular heart rhythm notifications accessible to more people.
