
What
You Should Know:
–
Digital health startup Kleva Health launches FDA authorized saliva at-home
COVID-19 test kit and raises $1.5 million in seed funding.
–
Kleva Health’s At-Home COVID-19 Saliva Test Kit will be available for orders on
its website, klevahealth.com, and will retail for US$149. Test results will be
reported on klevahealth.com and available within 48 hours after the test has
arrived at the lab.
Kleva Health, a San Francisco, CA-based digital health company, today announced the availability of its FDA Authorized at-home COVID-19 testing kits to offer Americans the choice to self-administer COVID-19 tests. In addition to the launch of at-home COVID-19 test kits, the company is also announcing $1.8M in seed funding from VCs including IMO Ventures, Human Longevity, and Performance Impact Venture Fund and Zenni Optical.
At-Home COVID-19 Saliva Test Kit

Kleva Health’s At-Home COVID-19 Saliva Test Kit uniquely
includes a Kleva Health saliva DNA/RNA collection device. This enables Kleva
Health to rapidly scale up its supply, without being dependent on distributors.
But a saliva test also enables the company to offer the ability to test for a
wider assortment of diseases and viruses – not just COVID-19.
Availability
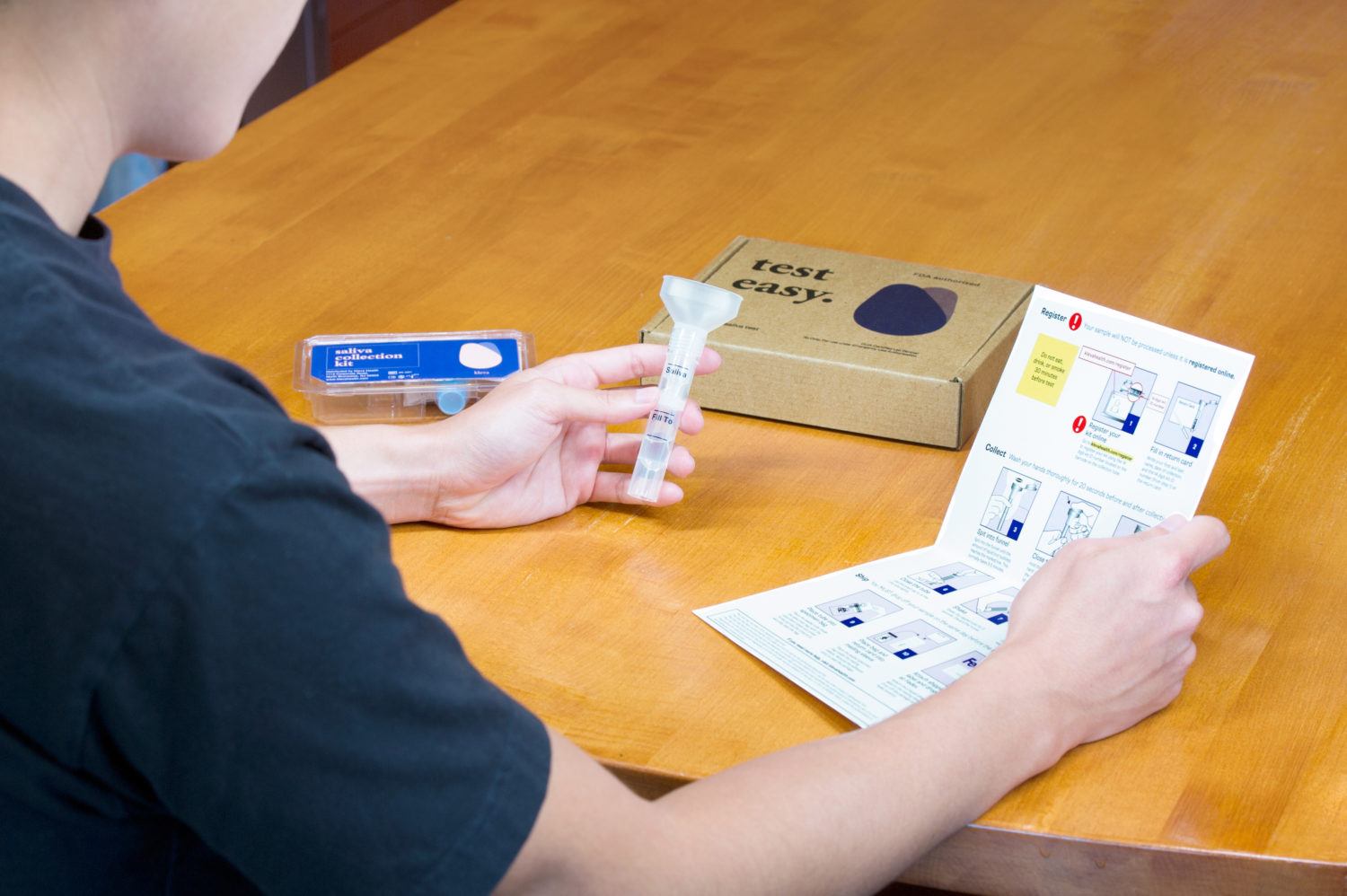
Kleva Health’s At-Home COVID-19 Saliva Test Kit will be
available for orders on its website, klevahealth.com, and will retail for US$149. Test results
will be reported on klevahealth.com
and available within 48 hours after the test has arrived at the lab.
Kleva Health Background
Kleva Health is founded by Harvard Business School
classmates and medical industry executives, David Yu, Bernie Siu and Kai Lim.
But the company started following a familiar story. Bernie Siu (Kleva Health’s
Chief Medical Officer and Doctor of Medicine from Stanford University’s School
of Medicine) had fallen ill from contract COVID-19 but discovered that getting
access to testing solutions ahead of his family reunion was easier said than
done. Not qualified for immediate testing at the time, Bernie believed that
there had to be a better solution. But he’s not alone. As many as 55.1% of
people do not know that at-home COVID-19 testing is an option for Americans,
according to the survey.
Responding to the New Normal
Fortunately, Kleva Health’s launch comes at an opportune
time. 55.4% of Americans are prepared for COVID-19 to be around longer than one
year. An additional 31.7% believe that COVID-19 will be around until a vaccine
is available. Not surprisingly, respondents overwhelmingly believe that
COVID-19 has made them realize the need for maintaining healthy habits,
including regular testing. As a digital health tracking company, Kleva Health
encourages healthy habits by offering Americans the convenience of at-home
tests, amid this new normal, and an online platform for keeping track of their
biomedical health.
“With COVID-19, many of us have a heightened awareness for not only our personal well-being, but also for those family members and friends around us. As the holidays are fast approaching, Kleva Health’s FDA authorized saliva-based COVID-19 self-testing kit, offers Americans the convenience and assurance that they can spend time with loved ones with peace of mind,” said David Yu, CEO and co-founder of Kleva Health. “But this product and the Kleva platform is just the start for us. Supported by our investors including IMO Ventures and the Human Longevity Fund, we plan to scale and introduce additional products that will make biomedical testing as easy and common as brushing one’s teeth.”
Future Plans
In addition,
Kleva Health will introduce in January 2021 a patent-pending and FDA authorized
next-generation COVID-19 saliva rapid testing kit that enables Americans to
self-test for the virus at home and read the results in just minutes.