What You Should Know:
– KPMG offers its data & analytics capabilities to
address emerging challenges tied to matching recovered patients with those in
clinical trials to receive convalescent plasma.
– KPMG’s capabilities can make the plasma donation process easier
and more efficient by automating the complex tasks and logistics associated
with the data collection, qualification and matching process by performing
statistical analytics, and using artificial intelligence (AI), virtualized
data, and blockchain to trace the plasma from donor to clinical trial
patient.
Recognizing the need for technologies to help advance the
response and recovery to the COVID-19
pandemic, KPMG LLP has built the technology
architecture for a solution that can match patients in clinical trials for
convalescent plasma therapy to recovered patients willing to donate their
antibodies.
Simplifying The Plasma Donation Process
Data & analytics can simplify the process of matching
patients who have recovered with patients that are undergoing clinical trials.
According to media
reports, the FDA paused the use of
convalescent plasma therapy for emergency cases, citing inconclusive data.
A federal
registry for clinical trials lists
54 U.S. studies of convalescent plasma therapy for treating COVID-19, with a
majority of them still recruiting patients.
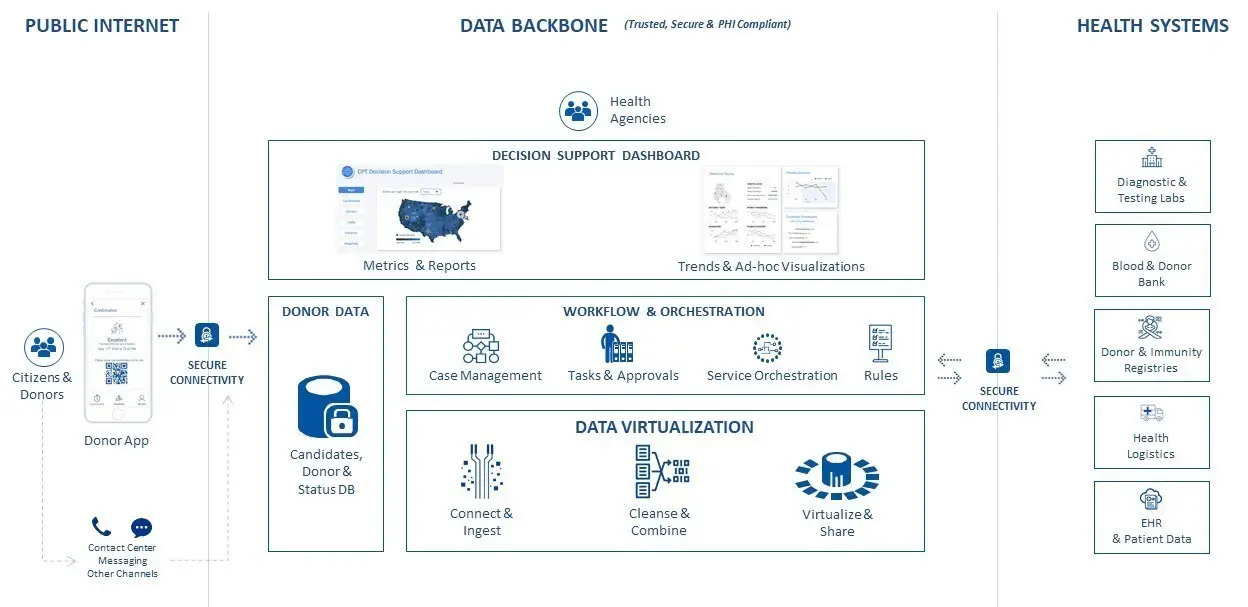
KPMG’s capabilities can make the plasma donation process easier
and more efficient by automating the complex tasks and logistics associated
with the data collection, qualification and matching process by performing
statistical analytics, and using artificial intelligence (AI), virtualized
data, and blockchain to trace the plasma from donor to clinical trial
patient. KPMG’s framework is designed to integrate third-party data
from blood banks, hospitals, and diagnostic labs and work with data backbones
and existing technology applications that are already in place, such as IT
infrastructure owned by governments and hospitals.
The technology can also be deployed for contact tracing or
monitoring vaccine use to help policy makers with population health and social
distancing decisions. KPMG introduced this technology as part of its
recently announced “Restarting America” initiative
to help organizations safely reopen workplaces.
“This solution is designed to help ease the burden on plasma donors, accelerate the COVID-19 plasma donation process and provide better visibility to health authorities, while helping facilitate the study of convalescent plasma,” said Bharat Rao, PhD, principal and leader of Data and Analytics for healthcare & life sciences at KPMG. “With the use of a mobile app, the data is better organized in a secure format, and reduces the duplication of requests and administrative overhead among healthcare organizations.”
