
Approximately 85% of rare diseases are genetic, offering significant opportunities to develop better treatments as our understanding of the human genome improves1. Advances in next-generation sequencing have shown that sequencing the whole genomes of large numbers of individuals in a standardized way can improve the diagnosis and treatment of patients with rare diseases.
Scientists have identified responsible genes for approximately 50% of the estimated 7000 rare diseases2. Pharmaceutical companies have long taken notice of this opportunity, developing a wave of therapies that are now entering the market.
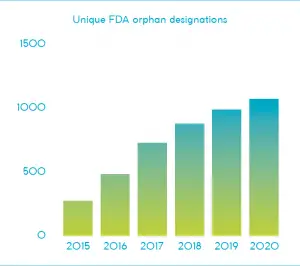
FDA data indicates that while the number of orphan designations has been increasing over the last half-decade, the number of unique indications has been decreasing3. Competition has become fiercer, with several companies moving into or doubling down in this space. Smaller biotechs, including Cerecor and Leadiant Biosciences, have also shown interest in niche ultra-rare diseases, defined as impacting fewer than one patient per 50,000 people4,5.
However, often rare disease patients are ignored by doctors, researchers, and the general public. Patients lack support across multiple points in their journey, including diagnosis, misdiagnosis, limited treatment options, lack of comprehensive information, and the absence of support groups.
Empowered patients taking control
The journey from symptom onset to diagnosis takes approximately 4.8 years for a rare disease6. Rare disease patients visit approximately seven healthcare professionals before receiving an accurate diagnosis6. Around 15% of patients seek a second opinion, and 5% have had unnecessary management and care2.
People affected by rare diseases are also impacted socio-economically due to the loss of jobs or having to leave education early, which then impacts their future career prospects.
As competition increases and patient populations become smaller, there is truly no better time than now to upskill in patient identification and acquisition
Patients, their families, and caregivers often face a sense of isolation and neglect, and this drives them to take charge of their conditions. As a result, they tend to handle their own care, form patient support groups, and self-identify funding and engagement opportunities7 – this makes them unique.
As competition increases and patient populations become smaller, there is truly no better time than now to upskill in patient identification and acquisition.
How do you find rare disease patients?
Concentrating on patient identification early in the clinical phases can drive brand loyalty and develop more effective tactics, to address unmet patient needs. It helps ensure that when the patient makes a treatment choice, they choose your brand.
Unlike other better-understood indications, rare disease referral pathways are convoluted. These illnesses are often more challenging to diagnose as the patient can present with multiple symptoms. A different physician will treat each symptom – as these physicians do not work together, it is harder to see the bigger picture, and connections can be missed.7 In many cases, the symptoms can also mimic other diseases, some of which are more common, and this leads to misdiagnoses.
Referral triggers may also be different. As rare diseases are often genetic, triggers can include the diagnosis of a family member, a family member’s suggestion, or even the patient’s self-referral. Mapping the extended stakeholder ecosystem will make it easier to determine drivers for a referral. Life science companies should use a systematic top-down approach, starting with centers of excellence and cascading to primary care physicians. By developing a visual journey map, brands can identify unmet needs and opportunities to make an impact.
Read the rest of the article in Delta magazine – rare disease unpacked
The post Making a big impact in small patient populations appeared first on .