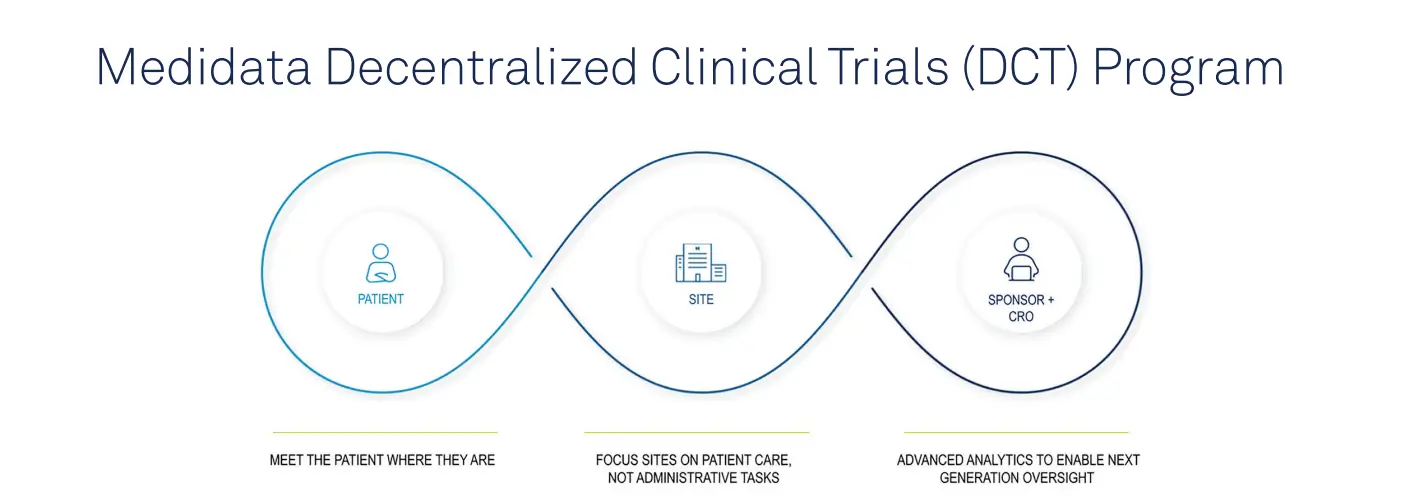
What You Should Know:
– Today Medidata, a Dassault Systèmes company, announced the launch of the Medidata Decentralized Clinical Trials (DCT) Program, the most comprehensive set of unified, secure technologies that enable full decentralization across the clinical trial continuum.
For the first time ever, drug, vaccine, and medical device developers (sponsors) and contract research organizations (CROs) can take advantage of the only platform offering on the market which combines:
– Technology and workflows to virtualize patient participation
– Oversight of patient safety and data quality
– Direct-to-patient services, including delivering study drugs to the home
The COVID-19 crisis has emphasized the pivotal role of technology in accelerating safe clinical trial development. In fact, Medidata technology helped to bring the Moderna COVID-19 vaccine through the full clinical trial life cycle in under a year. Medidata is now the only company providing a full suite of virtual capabilities to enable complete trial decentralization, encompassing both patient and site interactions.