One of specialties of Phil Baran’s group at Scripps the last few years has been electrosynthesis, which has a traditional hmm-interesting-turn-the-page reputation among most synthetic chemists that they’re trying to change. Photochemistry was in roughly the same category at one time, and has become much more mainstream (although it always had an advantage with lower barrier to entry). That field has made inroads through the advent of many new reactions, bond-forming processes that are hard to realize any other way, and that is surely the path forward for electrochemistry as well: provide relatively cheap, standardized equipment with easy setup and the promise of being able to do things you’ve never been able to do before.
Here’s a new paper from the group, now on ChemRxiv, that makes such a case. They demonstrate some weird effects of using square-waveform “rapid alternating polarity” instead of the traditional direct-current electrochemistry. These sorts of alternating-current applications have really not been explored much, and from this paper it appears that that neglect may have been a mistake. Take a look at this carbonyl reduction:
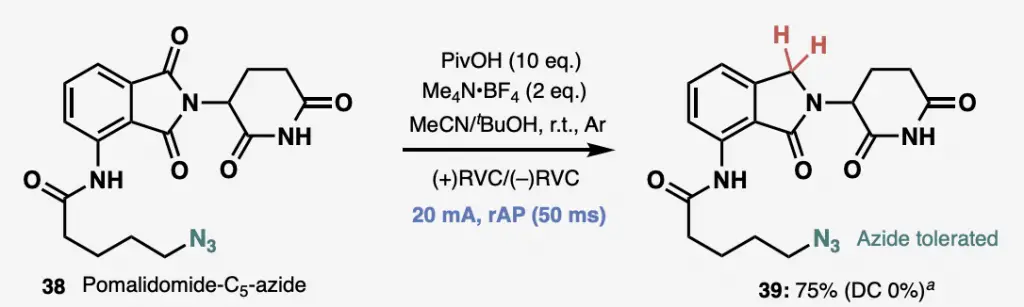
Protein degradation fans will recognize the pomalidomide building block at left, but I feel pretty sure that no one has one-shot conditions that will reduce one of the five carbonyls in the molecule selectively. You’ll note that you can’t even do that with traditional DC electrochemistry. This is one of the more dramatic examples from the paper; some of the other reactions on test molecules work fine either way, while some of them definitely show improved yields with the AC conditions. But this really gets the attention; you feel as if you have a magic wand when something like this works out.
The overall reduction is consistent with a (expected) mechanism that goes through a radical anion intermediate, followed by protonation. Running the reaction in deuterated methanol, for example, installs deuteriums on the reduced carbon. And the selectivities can be largely explained/predicted by the different reduction potentials of the carbonyls, which in turn can be approximated by their LUMO (lowest-unoccupied molecular orbital) coefficients. But when using the standard chemical reagents, we’re not really used to going direct to LUMOs to predict our reactions, because most of the carbonyl reducing agents are Lewis-acidic and complex the carbonyl oxygen as a first step. As the paper points out, synthetic chemists are used to having aldehydes as the fastest, cleanest carbonyl to reduce, but the paper shows an example where the phthalimide gets reduced in the presence of an aldehyde – which makes sense in terms of sheer reduction potential, but seems weird to one’s usual chemical intuition.
What’s weird is that switching to the square-waveform alternating current achieves this, while just plain direct current often doesn’t. The paper shows evidence that changing the frequency of the AC can have an effect on the reactions as well, depending on the kinetics of the possible reaction steps. Switching quickly enough between polarities can make the faster pathway possible before a slower step gets going, in other words. It seems that there are probably a lot of odd reactions waiting in this area, and no doubt folks in Baran’s group are zapping a whole range of molecules as we speak to find some new applications. Bring on the electric voodoo, say I – we need all the help we can get.
The post New Electron Tricks in Synthetic Chemistry first appeared on In the Pipeline.