We’ve got a lot of longstanding technical difficulties in the drug development business – things that we’d really like to be able to do, but can’t quite manage to find any general solutions to. Measuring drug concentrations inside cells (or parts of cells), that’s one. Predicting human toxicology for a new compound is another, for sure. And there’s also the problem of getting things past the blood-brain barrier that the body doesn’t want to let past.
For those outside the field, the actual BBB isn’t like some sort of steel plate around the bottom of the skull – it’s actually the capillaries that penetrate every region of the brain. It’s a rather heavily perfused organ, on the absolute scale – 15% of cardiac output going into about 2% of the body’s volume. But inner layer of cells in all those blood vessels are exceptionally closely packed, with help from astrocyte and pericyte cells. The end result is that entry into the brain’s fluid space is tightly guarded. Substances that fall outside of the preferred zones for molecular weight, size, and polarity generally don’t make it in at all (and the ones that do tend to have specific active transport pathways tailored for them, as witness the large number of glucose transporter proteins up there). Active transport is involved in the related problem of things being pumped right back out of the brain as well, even if they get in.
You wouldn’t want to just permeabilize the BBB overall, because most of that stuff that’s being excluded is being excluded for a reason. But we would like to get various drugs, proteins, antibodies, oligonucleotides and other species across in a more targeted fashion, and the literature over the last few decades is full of various solutions to that problem. I’ve written here before about attempts to use ultrasound to temporarily make the junctions looser, but as far as I know that technique is still rather experimental, if indeed it’s workable at all. Another popular technique has been to try to hitch a ride on those active transport proteins – if they could be persuaded to let in other species that have the right “passport” features hooked on to one end of them somehow, for example. These things can work, but I’m not aware of any that have really achieved wide usage, because every case is different.
There’s also been a lot of research into packaging such agents into new formulations, physical forms that have an easier time getting through – nanocarriers, for instance. The body’s own membrane-packaged exosomes have been researched for this purpose, too. I was talking the other day about lipid nanoparticles (LNPs), but those don’t seem to make it through. A new paper, though, has a trick that might allow them to. The team (from Tufts) synthesized lipid-like molecules that have neurotransmitter molecules (tryptamine, e.g.) attached to one end, via good old synthetic organic chemistry, and make lipid nanoparticles out of these. The resulting “neurotransmitter lipioid” (NT)-doped formulations actually seem to get taken up by the active transporters that recognize the neurotransmitters themselves.
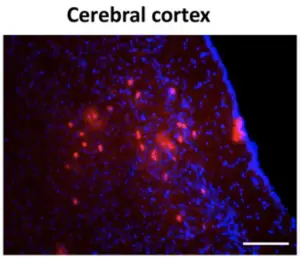
This is demonstrated with several interesting examples. Amphotericin B, for example, is a very important systemic antifungal drug of last resort, but it doesn’t cross the BBB. Formulated this way, though, it gets in. The paper goes on to show delivery of an antisense oligonucleotide to the Tau protein gene, and demonstrates that after such dosing that Tau mRNA does indeed go down in the brain tissue. A gene-editing Cre construct gets delivered as well. This is demonstrated in a mouse line that has a red fluorescent “Tomato” protein’s gene which is “floxed“, a technique that allows such a gene to be specifically targeted by Cre recombinase protein. In this case, it’s a “flox-stop-flox” system, which means that if the Cre recombinase is successfully delivered, the stop is removed and the tdTomato gene gets expressed. Sure enough, neurons of the treated animals fluoresce red when checked several days after treatment with the Cre formulation (cortex and cerebellum show more strongly than the hippocampus, for reasons unknown). There are some of the cells glowing at left – the team used this technique to evaluate several different formulations.
There are a lot of possibilities here. The paper takes on a number of different known lipid carrier formulations and just dopes them with their NT-lipidoid species, so it’s easy to try out a number of different ideas. And both the Cre and ASO examples demonstrate that at least some of these can go on to intracellular delivery once they get past the blood-brain barrier. I look forward to seeing how this gets developed!
