What You Should Know:
– Today Science 37 published a new report suggesting a momentous shift in the way that clinical trials are conducted.
– For the first time, more research sponsors and CROs are planning to run Agile/Hybrid Clinical Trials than ever before. Findings show that the vast majority or 77% of respondents plan to run an agile clinical trial in the next 12 months, compared to 59% for the previous 12 months.
Science 37 Holdings, Inc. (Nasdaq: SNCE) (“Science 37”), a LA-based pioneer of decentralization and Operating System for today’s more agile clinical trials™, has published new data suggesting that the clinical research industry has reached a pivotal shift in the way that clinical trials are conducted, as Sponsors and CROs report they plan to run more agile (or hybrid) clinical trials in 2022 than traditional, site-based studies.
Report Background
Findings are the result of a sentiment study among 127 senior clinical research executives in September and October 2021. Nearly eight of 10 respondents said they plan to run an agile clinical trial in the next 12 months. Conversely, just seven of 10 said they are planning to run a traditional, site-based study in the next 12 months.
Defining Agile Clinical Trials
Use of decentralized tools and methodologies is increasing. With these advances, patients will be able to participate from their home, a site, or a combination of home and site; and providers will be able to participate on or off-premises. This symbiotic clinical trial or the “agile clinical trial” requires: The ability to activate any provider and any patient, regardless of premises; a network of patient communities, telemedicine investigators, mobile nurses, and remote coordinators—all with a flexible Operating System to seamlessly navigate between on-site and off-site.
Key Findings
In addition to documenting this fundamental shift, the survey data also paints a vivid picture of the decentralized clinical trial (DCT) landscape for the year ahead, suggesting significant increases in DCT activity across multiple therapeutic areas and study phases. Among the findings:
– 46% of respondents expect to run an oncology study utilizing DCT in the next year, vs. 35% for the previous year—making oncology the most prevalent DCT category.
– Oncology, CNS, rare diseases, and immunology should see the biggest uptake in DCT use.
– The report suggests significant increases in DCT activity across Phase II, III, and IV studies.
– We also expect to see big increases in the deployment of most individual DCT components, particularly eConsent, telemedicine, mobile nurses, and remote sites in a hybrid model.
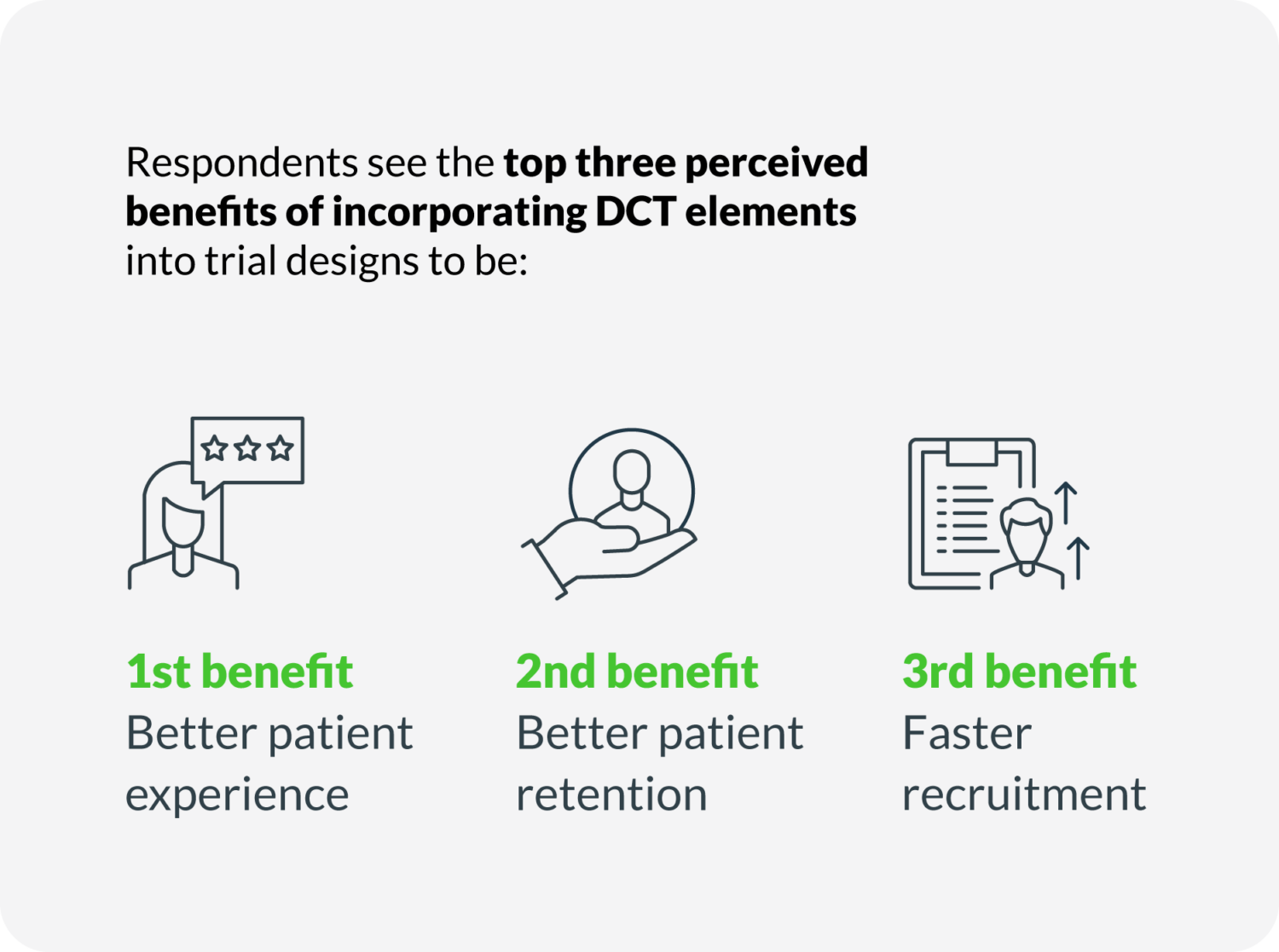
– The top-3 perceived benefits of DCT are better patient experience, better patient retention, and faster recruitment.
– The top-3 perceived challenges of DCT are integrating traditional sites with DCT, lack of in-house capabilities, and regulatory concerns.
“The power of increased decentralization is becoming undeniable as more sponsors benefit from faster enrollment, greater retention and increased diversity,” said David Coman, Chief Executive Officer at Science 37. “Leveraging the Science 37 Operating System, our customers are enabling much more agile clinical trial designs that realize these benefits while significantly reducing patient burden and delivering more universal access for patients and providers, anywhere.”