BACKGROUND:
Biomarkers are characteristics (physiologic & pathologic) that can be objectively assessed and evaluated as an indicator of normal biological process, pathogenic process, or pharmacological response to therapeutic alteration inside the body. Digital biomarkers help in the collection of physiological & behavioral measures of the consumer using digital tools that can be used to explain & predict health-related outcomes. These outcomes can differ by the means of disease explanations to the prediction of drug response thereby influencing the fitness behavior of a person.
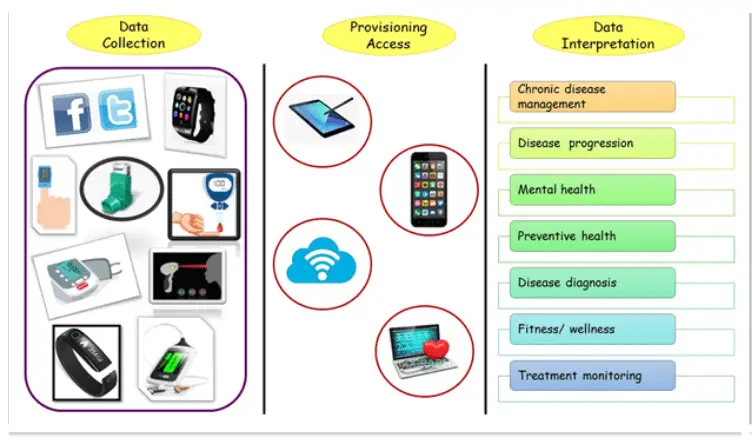
Figure 1: The role of digital biomarkers
In recent years companies such as Fitbit, Misfit, Jawbone, Apple health, MocaCare, Skeeper, etc which are into tracking sleep, fitness, blood pressure, etc. are growing fast. These companies become a substantial player in health & wellness, which are generating significant data not only about patients but also about individuals not on patient care. As per Market & Market data, the global marketplace for medical wearable devices is estimated to reach $12 billion by 2021. As these data-generating devices rise exponentially, the health care data also shows an astronomical growth rate. Patient-generated data using digital biomarkers provide new information in the healthcare industry.1
TYPES OF DIGITAL BIOMARKERS:
Digital biomarkers can be classified depending on the status of a particular measurement to a particular clinical outcome. Digital biomarker either replace non-digital biomarker (known as Approved), opens a new arena (Novel) or is a fusion/hybrid which on one hand substitute and on other hand unlocks new field (original).1
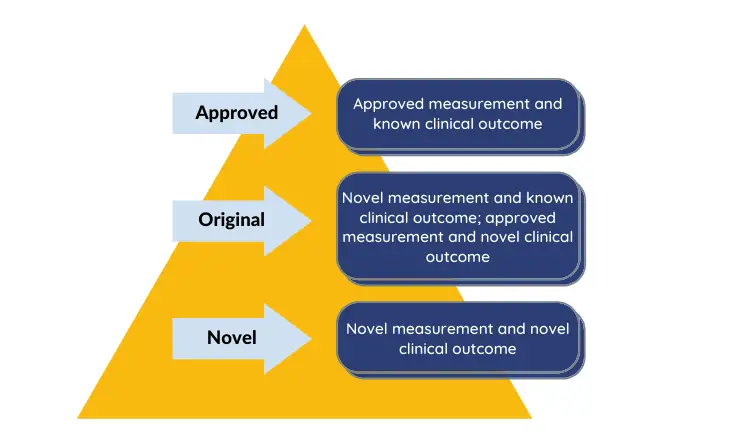
Figure 2: Classification of digital biomarkers
CURRENT APPLICATION OF DIGITAL BIOMARKERS:
Digital biomarkers can provide objective measurement which supports the diagnosis, prognosis, and measure therapeutic based outcomes. Currently, major pharma companies are running pilot studies to evaluate the feasibility of digital biomarker use. Roche has developed a Parkinson’s disease app to measure PD severity. Biogen in partnership with PatientsLikeMe developing an app to measure the physical activity of patients with multiple sclerosis whereas Neurotrack remotely assessed the cognitive ability of Alzheimer’s patients.
The outcome measures by using digital biomarkers in a clinical trial are limited but growing fast due to their precision & reliability. Recent years have observed the application of digital biomarkers in health areas such as cardiovascular disease, respiratory disorders, diabetes, neuropsychiatric disease, etc.
- Cardiovascular disease: Companies have engaged in the detection of atrial fibrillation for cardiovascular disease using algorithms based on smartwatch data. FDA has not approved algorithm-based data, but a digital biomarker for atrial fibrillation detection is available in conjunction with an approved EKG. Similarly, the study published in The New England Journal showed the use of accelerometer as the primary outcome measure in the treatment of congestive heart failure.2
- Sleep: Due to less validation requirement, a variety of digital biomarkers such as Fitbit’s sleep stages feature, MI Fit’ sleep score is commercially available. A study published in the journal Sleep in 2017, investigated the ability of wrist-worn tracker to estimate sleep stages in a normal adult. The result of the study demonstrated that the use of these devices can be used to track stages of sleep with reasonable accuracy in normal adults. These devices can simplify sleep research and will help to increase public awareness about sleep issues.3
- Respiratory Disorders: Smart inhalers for asthma and COPD consist of sensors that are attached to inhalers and monitor the use of medication. An objective assessment of inhaler adherence determined by obtaining the date, time & number of device actuation. These smart inhalers are Bluetooth-enabled and linked wirelessly with computers, tablets, or smartphones for automatic data transfer from inhalers. The companion mobile application gathers, interprets data & sends processed information to the healthcare professional. One of such FDA-approved applications developed by Propeller health known as Propeller health system which identifies the environmental triggers for patients with asthma, COPD & other respiratory conditions. It includes a sensor attached to a rescue inhaler or to a controller inhaler medication for effective management of the condition.4 Teva, another pharma company also launched FDA approved smart inhaler, Digihaler device in 2019 in the respiratory portfolio.5
- Diabetes: Diabetes is one of the major metabolic disorder which affect the large population worldwide. It always remained a highly innovative area in terms of digital therapeutics for pharma companies. San Francisco based company Omada Health; brought evidence-based digital therapeutics in the area of pre-diabetes/diabetes. The company offers a special online diabetes prevention program known as “Prevention” in association with the National Institutes of Health (NIH) which provides information regarding how diet and exercise reduce the chances of developing diabetes. During the program, each participant received a wireless digital scale and pedometer and interact with a personal health coach for daily feedback using an online platform. Results showed that participants lost 4.7% more body weight than an average weight loss of 2.4% for personal diabetes prevention programs.6 Merck & amazon partnership announced they are aiming to develop voice-enabled solutions for people with diabetes using Amazon’s Echo dot & Alexa.7
- Neuropsychiatric Disease: Different research programs are under progress in the area of neuropsychiatric disease. Researchers started examining wearables, accelerometers, and smartwatch devices in adults with Parkinson’s disease, Alzheimer’s disease, and dementia. Roche has developed a Parkinson disease application to capture voice-related information for obtaining early symptoms associated with Parkinson’s. Application collect voice data on phone & calculate stability when user hold devices.8
Neurotrack developed digital online test for detection of cognitive deficit in Alzheimer’s patient. The test is inexpensive & have potential of easy to use approach for detection of Alzheimer before development of clinical symptom. The test assessment are takes repeatedly so that user can track how memory changes over the time.9
CHALLENGES TO BE OVERCOME IN NEAR FUTURE10
- Standardized solutions: To compare & evaluate the results obtained during the studies by using digital biomarkers, standardized solutions are needed. During clinical trials, the use of standardized procedures ensures similar sampling in every case and therefore results are comparable with another achievement. In the case of consumer-generated data, it is not evident. The measurement carried out by smartwatches or trackers could be completely different in each situation.
- Reliability: As not all digital biomarkers are FDA or CE approved, the reliability of the instrument is questionable.
- Measurement issue: The measurement details can be entirely distinct as individuals can measure (for eg ECG) with different tools at home.
- Privacy or regulatory issue: Ensuring privacy and autonomy is of prime importance as digital biomarkers send measured data directly to the company or researcher. The data use agreement for digital biomarker should be thoroughly followed and must be monitored by government authority.
CONCLUSION:
Continuous monitoring of healthy as well as diseased persons using digital biomarkers will bring new data to a healthcare professional on regular basis. In the past, many incidences occurred where the use of new technology leads to a paradigm shift in the medical field which enabled new questions and novel insights in the healthcare system. The use of digital biomarkers will not cause a shift in the medical paradigm but it will offer novel ways for the measurement of health status which provides observations and perspectives into a disease that were unavailable before. Digital biomarkers will help to provide supplementary information & augment conclusions obtained from traditional biomarkers. The detailed measurement along with accurate & precise evaluation from molecular characterization of the disease using digital biomarkers will help to redefine diagnosis and classification of disease. The molecular profiling of disease with the help of digital biomarker can further support precision medicine & leads to innovative treatment in different disease. Finally, digital biomarkers not only provide information about what we know about the disease but also about understanding of their own health.
REFERENCES:
- Traditional and Digital Biomarkers: Two Worlds Apart?
- Accelerometer-Measured Daily Activity in Heart Failure With Preserved Ejection Fraction
- 0068 ESTIMATION OF SLEEP STAGES USING CARDIAC AND ACCELEROMETER DATA FROM A WRIST-WORN DEVICE
- Propeller
- Teva Announces FDA Approval of First and Only Digital Inhaler with Built-In Sensors – ProAir Digihaler (albuterol sulfate 117 mcg) Inhalation Powder
- Omadahealth
- Merck aims to put Amazon’s Alexa to work on voice-enabled diabetes tools
- Roche will use the app to track Parkinson’s symptoms in drug trial
- Neurotrack
- Digital technologies as biomarkers, clinical outcomes assessment, and recruitment tools in Alzheimer’s disease clinical trials.
About Co-Author:

Dr. Sunaina Anand, Pharm. D is a Clinical Pharmacist. She currently serves as Medical Affairs Executive in IntelliMed Healthcare Solutions. She previously interned in Tata Memorial Hospital and Columbia Asia Hospital, Bengaluru.
The post ViewPoints Article: Digital Biomarkers first appeared on PharmaShots.
