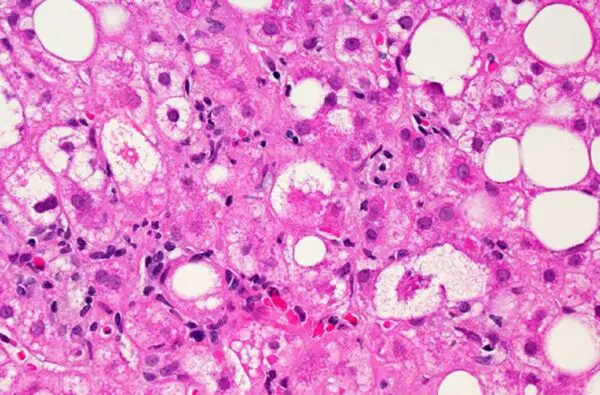
Two years after the stinging FDA rejection of its drug for the fatty liver disease NASH, Intercept Pharmaceuticals has more safety and efficacy data from a pivotal study that could support resubmission of a new drug application. The biotech said it will meet with the FDA later this month.