Shots:
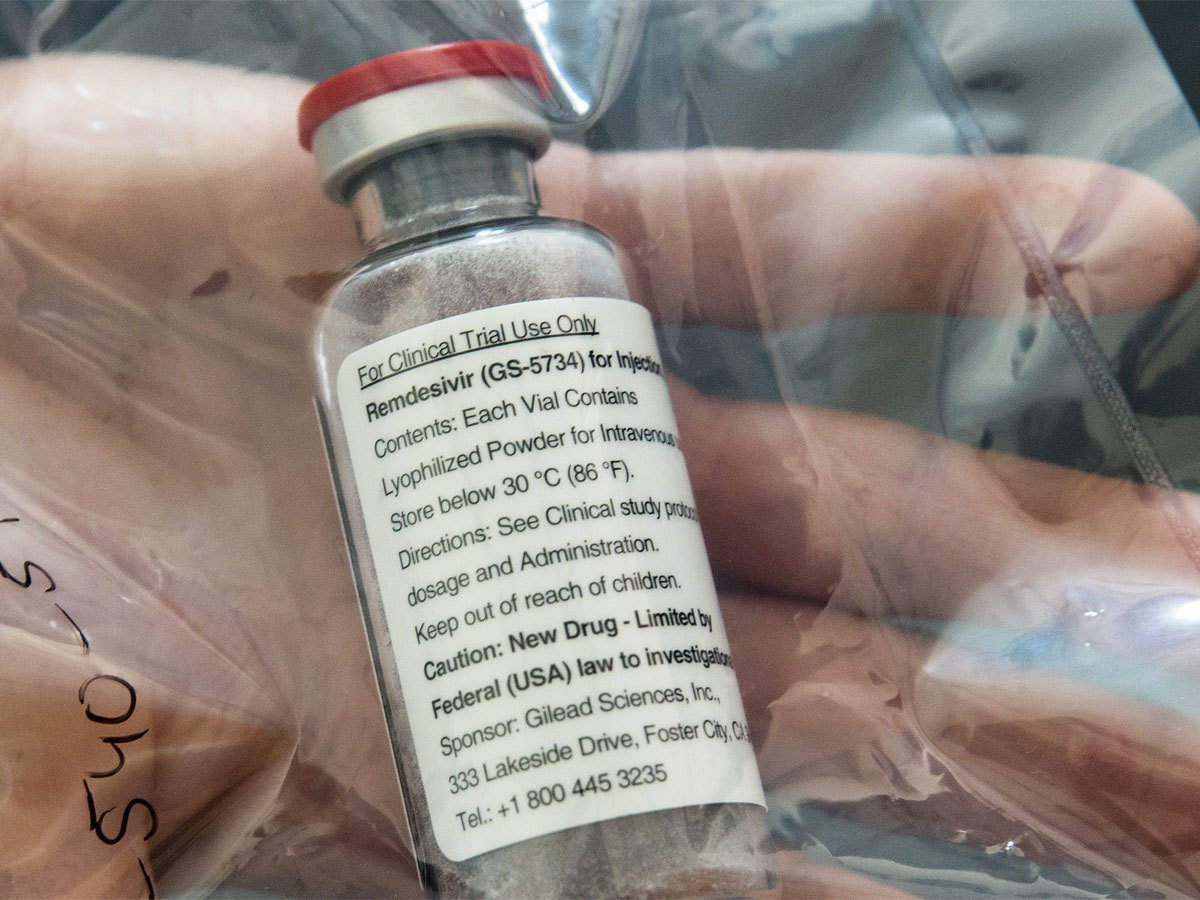
- Mylan launches Desrem (remdesivir) addressing the unmet needs amid COVID-19. The drug is approved for the treatment of suspected or laboratory-confirmed incidences of COVID-19 in adults and children hospitalized with severe symptoms
- Mylan will manufacture Desrem in its state-of-the-art injectable facility in Bangalore, India, and other export markets where Mylan has received a license from Gilead for the commercialization of remdesivir
- The company has released the first batch of its generic remdesivir and will continue to increase its supply across the country in the wake of the rising demand for the drug. Patients and HCPs can access information about the availability of Desrem in India through Mylan’s 24/7 national helpline number, +91.78299.80066
Click here to read full press release/ article | Ref: Mylan | Image: Ommcom News
