So let’s take a break today by looking at some compounds that, to a good approximation, many synthetic organic chemists would agree shouldn’t even exist. Yep, it’s time for a dive into Weirdo Natural Products, as I do every so often around here. I’ll point out some of the more ridiculous features for the non-chemists in the crowd, but some of the structures may well speak for themselves (!)
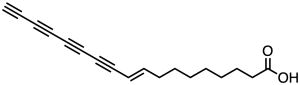
First up is protogenin A, a subject of this recent paper. That’s a “poly-yne” molecule of course, with that tail of acetylene groups stretching off, and you will have to search hard to find anyone who’s made something like that synthetically. And if you’ve made one, it probably didn’t stick around – such things are not so stable, and start isomerizing, polymerizing, oxidizing, and just generally going to pieces on you pretty rapidly. The paper notes the difficulty of isolating these from the organisms that make them, for just those reasons. Presumably the reactivity is part of the reason for their existence; they’re believed to be bacterial defense compounds. Well, if not outright offensive warfare, because bacteria sure aren’t above those tactics, either. They’re good enough at that that the microorganisms producing these compounds are actually beneficial when growing around plants, because they’re particularly effective against oomycetes, a fungal-like class of organisms that cause some particularly nasty plant diseases.
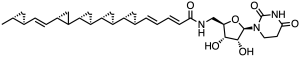
In a broadly similar vein, we have jawsamycin, at right. Yep, five chiral cyclopropane rings, interrupted for some reason by an alkene that didn’t get cyclopropanated. This is not a very obvious structure to figure out from spectroscopy, partly because it’s so repetitive and partly because it’s so hard to believe (although you’re certainly notice quickly from the NMR spectra that you’re facing a big pile of cyclopropanes). But there are several of these poly-cyclopropyl things out there in the natural products world. Admittedly, one would qualify as “quite a few”, by most standards. The rings are formed (in this case) by odd iron-sulfur enzymes that make a ylide intermediate out of S-adenosylmethionine. It has strong antifungal activity, targeting a key enzyme in the construction of the fungal cell wall. And if you haven’t noticed the name, go back, look at it again, and think about movies from the 1970s. Yep, chemist humor strikes again!
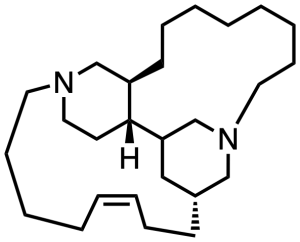
Halicyclamine B, at right, is not a lot of fun to draw, and if you have some spare time you can probably do a better job of it than I did. But two six-membered rings fused into a 13-membered ring and a 15-membered ring, that’s not going to flow right onto the page no matter what. Marine sponges make a whole list of such things, some of which will feature in future installments of this series. Their biological activities are wide-ranging, and my impression is that many of them have not been convincingly assigned to any given target. If you have access to that book chapter linked above, you can brace yourself for some seriously distorted bonds as the authors attempt to draw all these things in a reasonably compact and understandable form. It is clear that natural products are under no obligation to present themselves in structures that can be rendered intelligible on the page.
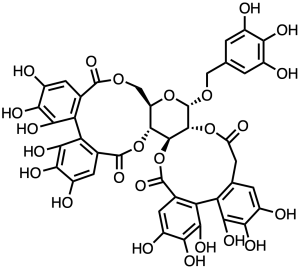
OK, enough nitrogens for a moment. Take a look at sanguiin H-11, which is nothing if not overwhelmingly rich in oxygen functional groups. That’s a glucose buried in the middle of it, and if you know your plant natural products you’ll recognize a bunch of gallic acid-derived polyphenols around the margin of the thing. Fifteen phenols on one molecule enough for you? There’s a large family of such things (glucose surrounded by more phenolic stuff than the mind can take in), and their structural chemistry will make your ears ring and your eyes bulge. Here, try it out. This is the sort of thing you get when you open up the box labeled “tannin molecules”, and most people close it right back up again. This one has strong effects on oxidative stress pathways in cells, probably through inhibiting activation of MAP kinase. But how it does that is a mystery to me! I’m just impressed that such a beast has cellular activity at all: show this thing to most medicinal chemists and you’ll get the sort of look that Clint Eastwood gives a lineup of villains as they come riding over a dusty ridge in the distance.
The post Weird Natural Product Time Again first appeared on In the Pipeline.
